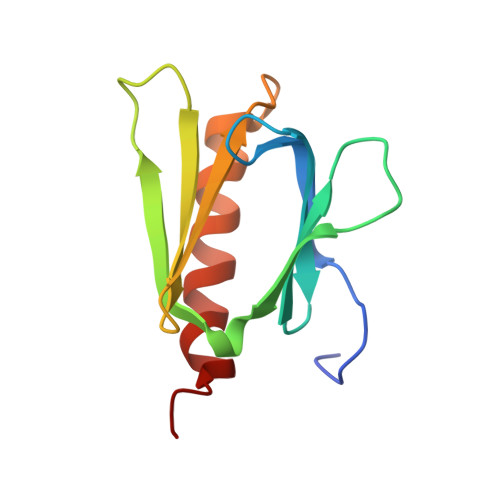
Structural Insight into the Mechanism of TFIIH Recognition by the Acidic String of the Nucleotide Excision Repair Factor XPC.
Okuda, M., Kinoshita, M., Kakumu, E., Sugasawa, K., Nishimura, Y.(2015) Structure 23: 1827-1837
- PubMed: 26278177
- DOI: https://doi.org/10.1016/j.str.2015.07.009
- Primary Citation of Related Structures:
2RVB - PubMed Abstract:
In global genome repair (GGR), XPC detects damaged nucleotides and recruits TFIIH complex. The small acidic region of XPC binds to the pleckstrin homology (PH) domain of TFIIH subunit p62; however, the recognition mechanism remains elusive. Here, we use nuclear magnetic resonance to present the tertiary structure of XPC bound to the PH domain. The XPC acidic region forms a long string stabilized by insertion of Trp133 and Val136 into two separate hollows of the PH domain, coupled with extensive electrostatic contacts. Analysis of several XPC mutants revealed that particularly Trp133 is essential for binding to the PH domain. In cell lines stably expressing mutant XPC, alanine substitution at Trp133 or Trp133/Val136 compromised UV resistance, recruitment of TFIIH to DNA damage, and removal of UV-induced photoproducts from genomic DNA. These findings show how TFIIH complex is recruited by XPC to damaged DNA, advancing our understanding of the early stage of GGR.
- Graduate School of Medical Life Science, Yokohama City University, 1-7-29 Suehiro-cho, Tsurumi-ku, Yokohama 230-0045, Japan.
Organizational Affiliation: