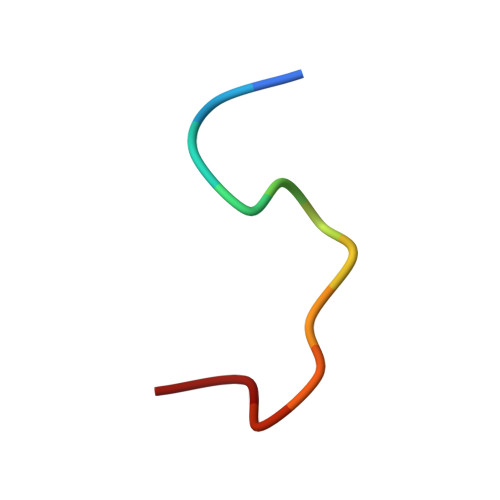
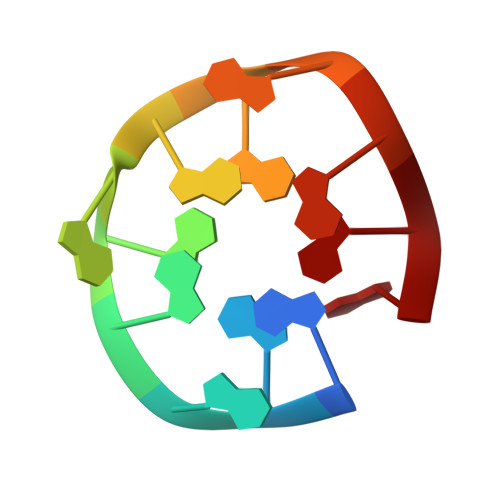
Anti-prion activity of an RNA aptamer and its structural basis
Mashima, T., Nishikawa, F., Kamatari, Y.O., Fujiwara, H., Saimura, M., Nagata, T., Kodaki, T., Nishikawa, S., Kuwata, K., Katahira, M.(2013) Nucleic Acids Res 41: 1355-1362
- PubMed: 23180780
- DOI: https://doi.org/10.1093/nar/gks1132
- Primary Citation of Related Structures:
2RSK - PubMed Abstract:
Prion proteins (PrPs) cause prion diseases, such as bovine spongiform encephalopathy. The conversion of a normal cellular form (PrP(C)) of PrP into an abnormal form (PrP(Sc)) is thought to be associated with the pathogenesis. An RNA aptamer that tightly binds to and stabilizes PrP(C) is expected to block this conversion and to thereby prevent prion diseases. Here, we show that an RNA aptamer comprising only 12 residues, r(GGAGGAGGAGGA) (R12), reduces the PrP(Sc) level in mouse neuronal cells persistently infected with the transmissible spongiform encephalopathy agent. Nuclear magnetic resonance analysis revealed that R12, folded into a unique quadruplex structure, forms a dimer and that each monomer simultaneously binds to two portions of the N-terminal half of PrP(C), resulting in tight binding. Electrostatic and stacking interactions contribute to the affinity of each portion. Our results demonstrate the therapeutic potential of an RNA aptamer as to prion diseases.
- Institute of Advanced Energy, Kyoto University, Gokasho, Uji, Kyoto 611-0011, Japan.
Organizational Affiliation: