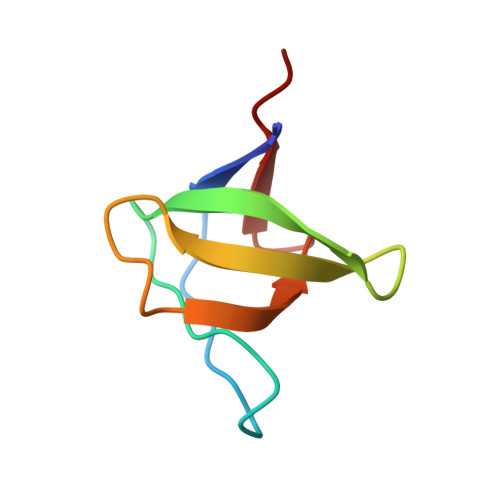
Structural basis of the recognition of the SAMP motif of adenomatous polyposis coli by the Src-homology 3 domain.
Kaieda, S., Matsui, C., Mimori-Kiyosue, Y., Ikegami, T.(2010) Biochemistry 49: 5143-5153
- PubMed: 20509626
- DOI: https://doi.org/10.1021/bi100563z
- Primary Citation of Related Structures:
2RQT, 2RQU - PubMed Abstract:
Elucidation of the basis of interactions between biological molecules is essential for the understanding of living systems. Src-homology 3 (SH3) domains play critical roles in interaction networks of proteins by recognizing a proline-rich sequence motif, PxxP. There are, however, several SH3 domains that specifically bind to polypeptide chains without the conventional recognition sequence. The SH3 domain of DDEF1 associates with the SAMP motifs of the adenomatous polyposis coli (APC) tumor suppressor. The SAMP motifs are indispensable for the normal function of APC in tumor suppression. Here we present the structural basis of the interaction between the DDEF1-SH3 domain and the APC-SAMP motifs. We determined the solution structures of the DDEF1-SH3 domain both in a free state and in a complex with APC-SAMP. As the affinity of the interaction was not sufficiently high for the determination of the complex structure in solution by conventional methods, we utilized a fusion protein of the DDEF1-SH3 domain and APC-SAMP. The structures revealed that the SAMP motif adopts a class II polyproline type II helix even though it does not contain the PxxP motif and that a characteristically large hydrophobic pocket of the SH3 domain confers high selectivity to the interaction. Furthermore, investigation into the backbone dynamics of the free and bound systems by NMR spin relaxation experiments demonstrated that the DDEF1-SH3 domain exhibits high flexibility at the peptide recognition site in the absence of the ligand and that most residues of the APC-SAMP motif display extensive local motions even in the stable complex.
- Institute for Protein Research, Osaka University, 3-2 Yamadaoka, Suita, Osaka 565-0871, Japan.
Organizational Affiliation: