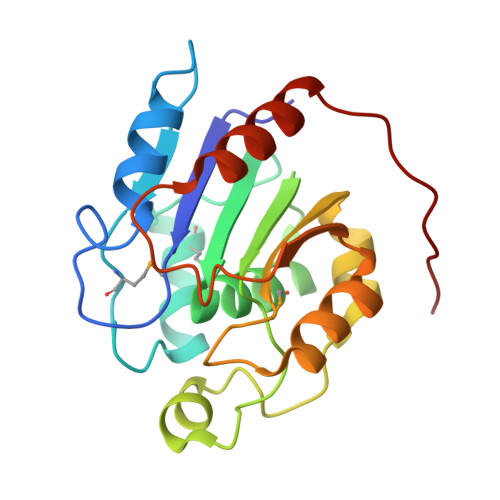
Crystal structure of human retinoblastoma binding protein 9.
Vorobiev, S.M., Su, M., Seetharaman, J., Huang, Y.J., Chen, C.X., Maglaqui, M., Janjua, H., Proudfoot, M., Yakunin, A., Xiao, R., Acton, T.B., Montelione, G.T., Tong, L.(2008) Proteins 74: 526-529
- PubMed: 19004028
- DOI: https://doi.org/10.1002/prot.22278
- Primary Citation of Related Structures:
2QS9 - Department of Biological Sciences, Northeast Structural Genomics Consortium, Columbia University, New York, New York 10027, USA.
Organizational Affiliation: