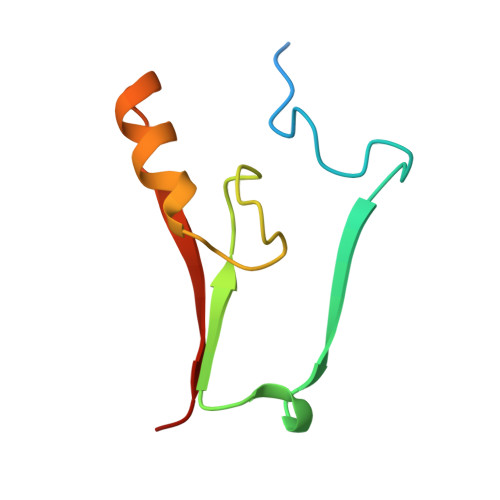
MitoNEET is a uniquely folded 2Fe 2S outer mitochondrial membrane protein stabilized by pioglitazone.
Paddock, M.L., Wiley, S.E., Axelrod, H.L., Cohen, A.E., Roy, M., Abresch, E.C., Capraro, D., Murphy, A.N., Nechushtai, R., Dixon, J.E., Jennings, P.A.(2007) Proc Natl Acad Sci U S A 104: 14342-14347
- PubMed: 17766440
- DOI: https://doi.org/10.1073/pnas.0707189104
- Primary Citation of Related Structures:
2QH7 - PubMed Abstract:
Iron-sulfur (Fe-S) proteins are key players in vital processes involving energy homeostasis and metabolism from the simplest to most complex organisms. We report a 1.5 A x-ray crystal structure of the first identified outer mitochondrial membrane Fe-S protein, mitoNEET. Two protomers intertwine to form a unique dimeric structure that constitutes a new fold to not only the approximately 650 reported Fe-S protein structures but also to all known proteins. We name this motif the NEET fold. The protomers form a two-domain structure: a beta-cap domain and a cluster-binding domain that coordinates two acid-labile 2Fe-2S clusters. Binding of pioglitazone, an insulin-sensitizing thiazolidinedione used in the treatment of type 2 diabetes, stabilizes the protein against 2Fe-2S cluster release. The biophysical properties of mitoNEET suggest that it may participate in a redox-sensitive signaling and/or in Fe-S cluster transfer.
- Department of Physics, University of California at San Diego, La Jolla, CA 92093, USA.
Organizational Affiliation: