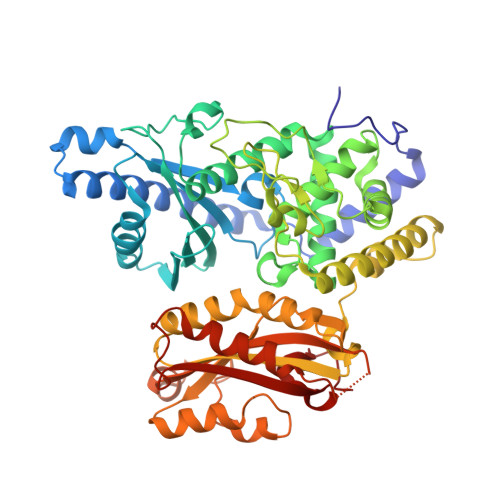
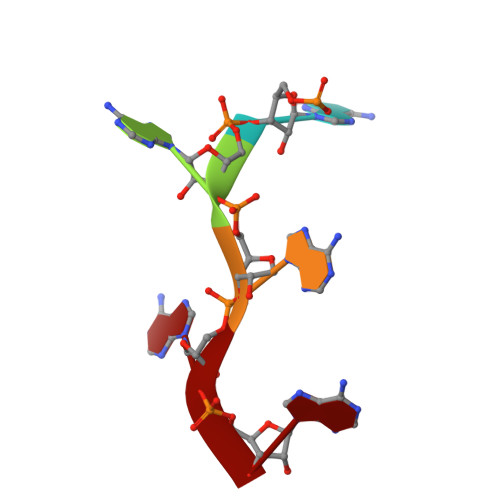
Mechanism of poly(A) polymerase: structure of the enzyme-MgATP-RNA ternary complex and kinetic analysis.
Balbo, P.B., Bohm, A.(2007) Structure 15: 1117-1131
- PubMed: 17850751
- DOI: https://doi.org/10.1016/j.str.2007.07.010
- Primary Citation of Related Structures:
2Q66 - PubMed Abstract:
We report the 1.8 A structure of yeast poly(A) polymerase (PAP) trapped in complex with ATP and a five residue poly(A) by mutation of the catalytically required aspartic acid 154 to alanine. The enzyme has undergone significant domain movement and reveals a closed conformation with extensive interactions between the substrates and all three polymerase domains. Both substrates and 31 buried water molecules are enclosed within a central cavity that is open at both ends. Four PAP mutants were subjected to detailed kinetic analysis, and studies of the adenylyltransfer (forward), pyrophosphorolysis (reverse), and nucleotidyltransfer reaction utilizing CTP for the mutants are presented. The results support a model in which binding of both poly(A) and the correct nucleotide, MgATP, induces a conformational change, resulting in formation of a stable, closed enzyme state. Thermodynamic considerations of the data are discussed as they pertain to domain closure, substrate specificity, and catalytic strategies utilized by PAP.
- Department of Biochemistry, Tufts School of Medicine, 136 Harrison Avenue, Boston, MA 02111, USA.
Organizational Affiliation: