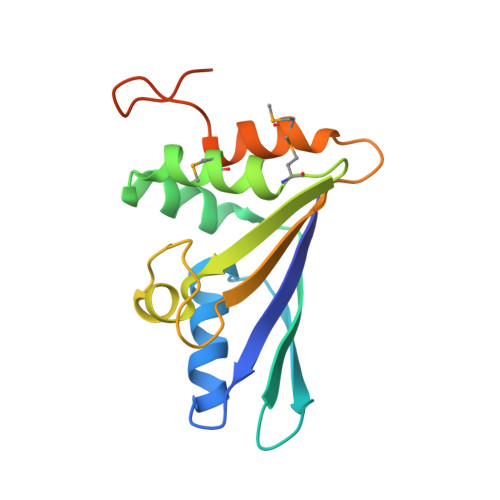
UPF201 archaeal specific family members reveal structural similarity to RNA-binding proteins but low likelihood for RNA-binding function.
Rao, K.N., Burley, S.K., Swaminathan, S.(2008) PLoS One 3: e3903-e3903
- PubMed: 19079550
- DOI: https://doi.org/10.1371/journal.pone.0003903
- Primary Citation of Related Structures:
2NRQ, 2NWU, 2OGK, 2PZZ - PubMed Abstract:
We have determined X-ray crystal structures of four members of an archaeal specific family of proteins of unknown function (UPF0201; Pfam classification: DUF54) to advance our understanding of the genetic repertoire of archaea. Despite low pairwise amino acid sequence identities (10-40%) and the absence of conserved sequence motifs, the three-dimensional structures of these proteins are remarkably similar to one another. Their common polypeptide chain fold, encompassing a five-stranded antiparallel beta-sheet and five alpha-helices, proved to be quite unexpectedly similar to that of the RRM-type RNA-binding domain of the ribosomal L5 protein, which is responsible for binding the 5S- rRNA. Structure-based sequence alignments enabled construction of a phylogenetic tree relating UPF0201 family members to L5 ribosomal proteins and other structurally similar RNA binding proteins, thereby expanding our understanding of the evolutionary purview of the RRM superfamily. Analyses of the surfaces of these newly determined UPF0201 structures suggest that they probably do not function as RNA binding proteins, and that this domain specific family of proteins has acquired a novel function in archaebacteria, which awaits experimental elucidation.
- Biology Department, Brookhaven National Laboratory, Upton, New York, United States of America.
Organizational Affiliation: