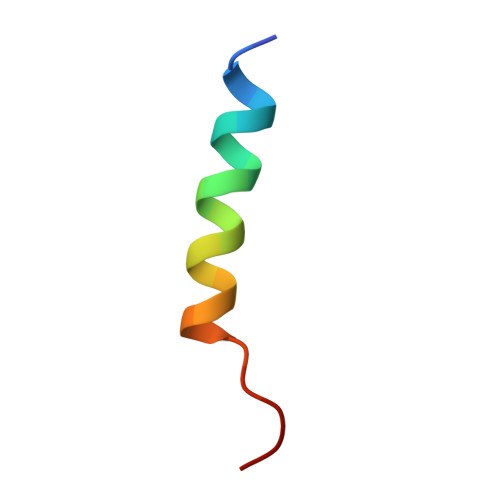
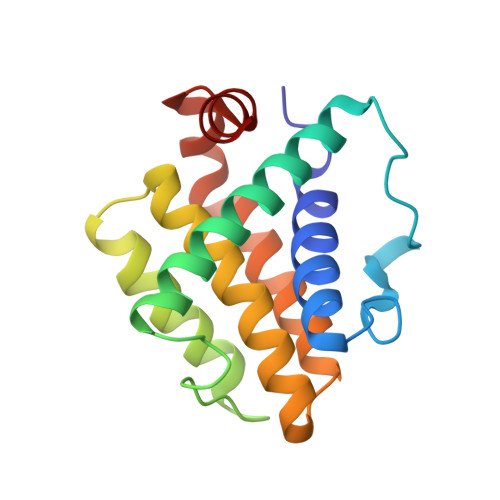
Molecular Basis of Bcl-xL's Target Recognition Versatility Revealed by the Structure of Bcl-xL in Complex with the BH3 Domain of Beclin-1.
Feng, W., Huang, S., Wu, H., Zhang, M.(2007) J Mol Biology 372: 223-235
- PubMed: 17659302
- DOI: https://doi.org/10.1016/j.jmb.2007.06.069
- Primary Citation of Related Structures:
2PON - PubMed Abstract:
Beclin-1, originally identified as a Bcl-2 binding protein, is an evolutionarily conserved protein required for autophagy. The direct interaction between Beclin-1 and Bcl-2 or Bcl-xL provides a potential convergence point for apoptosis and autophagy, two programmed cell death processes. Given the functional significance of the interaction between Beclin-1 and Bcl-2/Bcl-xL, we performed detailed biochemical and structural characterizations of this interaction. We demonstrated that the Bcl-xL-binding domain of Beclin-1 contains a BH3 domain. Therefore, Beclin-1 is a new member of the BH3-only family proteins. The structure of Bcl-xL in complex with the Beclin-1 BH3 domain was determined at high resolution by NMR spectroscopy. Although similar to other known BH3 domains, the Beclin-1 BH3 domain displays its own distinct features in the complex with Bcl-xL. Systematic analysis of all known Bcl-xL/BH3 domain complexes helped us to identify the molecular basis underlying the capacity of Bcl-xL to recognize diverse target sequences.
- Department of Biochemistry, Molecular Neuroscience Center, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, P. R. China.
Organizational Affiliation: