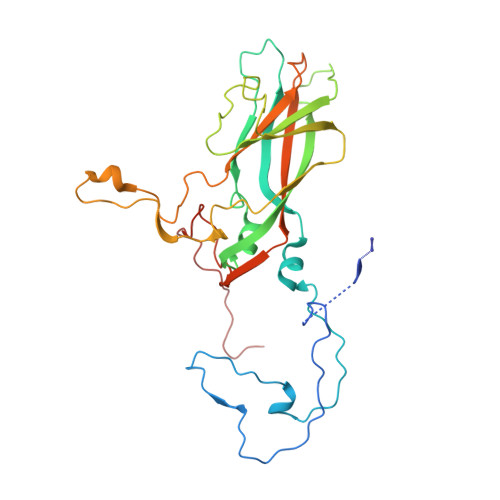
Structural factors that control conformational transitions and serotype specificity in type 3 poliovirus
Filman, D.J., Syed, R., Chow, M., Macadam, A.J., Minor, P.D., Hogle, J.M.(1989) EMBO J 8: 1567-1579
- PubMed: 2548847
- DOI: https://doi.org/10.1002/j.1460-2075.1989.tb03541.x
- Primary Citation of Related Structures:
2PLV - PubMed Abstract:
The three-dimensional structure of the Sabin strain of type 3 poliovirus has been determined at 2.4 A resolution. Significant structural differences with the Mahoney strain of type 1 poliovirus are confined to loops and terminal extensions of the capsid proteins, occur in all of the major antigenic sites of the virion and typically involve insertions, deletions or the replacement of prolines. Several newly identified components of the structure participate in assembly-dependent interactions which are relevant to the biologically important processes of viral assembly and uncoating. These include two sites of lipid substitution, two putative nucleotides and a beta sheet formed by the N-termini of capsid proteins VP4 and VP1. The structure provides an explanation for the temperature sensitive phenotype of the P3/Sabin strain. Amino acids that regulate temperature sensitivity in type 3 poliovirus are located in the interfaces between promoters, in the binding site for a lipid substituent and in an assembly-dependent extended beta sheet that stabilizes the association of pentamers. Several lines of evidence indicate that these structural components also control conformational transitions at various stages of the viral life cycle.
- Department of Molecular Biology, Research Institute of Scripps Clinic, La Jolla, CA 92037.
Organizational Affiliation: