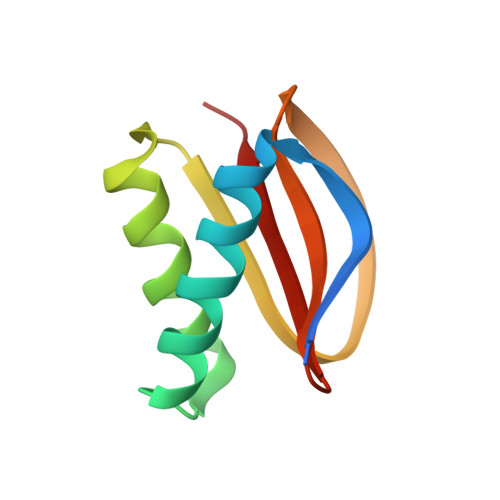
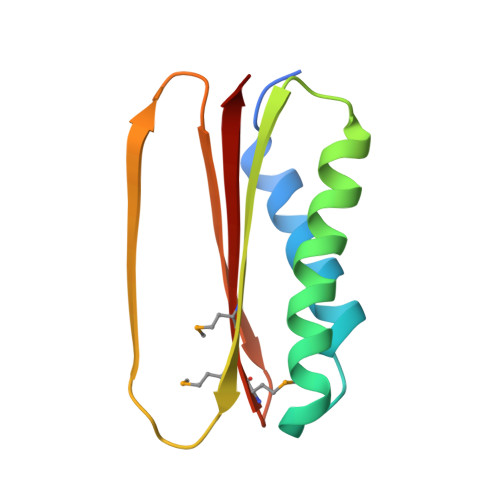
Structural and thermodynamic characterization of a cytoplasmic dynein light chain-intermediate chain complex
Williams, J.C., Roulhac, P.L., Roy, A.G., Vallee, R.B., Fitzgerald, M.C., Hendrickson, W.A.(2007) Proc Natl Acad Sci U S A 104: 10028-10033
- PubMed: 17551010
- DOI: https://doi.org/10.1073/pnas.0703614104
- Primary Citation of Related Structures:
2PG1 - PubMed Abstract:
Cytoplasmic dynein is a microtubule-based motor protein complex that plays important roles in a wide range of fundamental cellular processes, including vesicular transport, mitosis, and cell migration. A single major form of cytoplasmic dynein associates with membranous organelles, mitotic kinetochores, the mitotic and migratory cell cortex, centrosomes, and mRNA complexes. The ability of cytoplasmic dynein to recognize such diverse forms of cargo is thought to be associated with its several accessory subunits, which reside at the base of the molecule. The dynein light chains (LCs) LC8 and TcTex1 form a subcomplex with dynein intermediate chains, and they also interact with numerous protein and ribonucleoprotein partners. This observation has led to the hypothesis that these subunits serve to tether cargo to the dynein motor. Here, we present the structure and a thermodynamic analysis of a complex of LC8 and TcTex1 associated with their intermediate chain scaffold. The intermediate chains effectively block the major putative cargo binding sites within the light chains. These data suggest that, in the dynein complex, the LCs do not bind cargo, in apparent disagreement with a role for LCs in dynein cargo binding interactions.
- Department of Biochemistry and Molecular Biology, Thomas Jefferson University, Philadelphia, PA 19107, USA.
Organizational Affiliation: