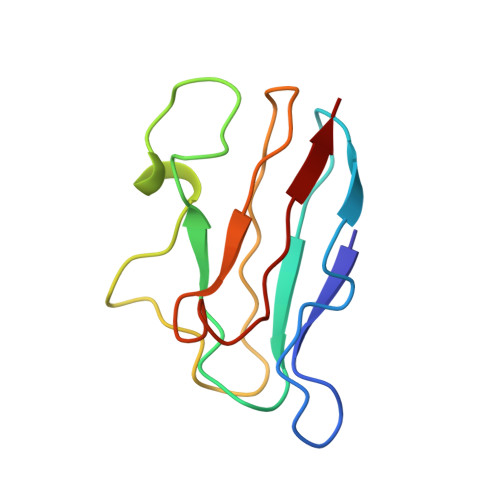
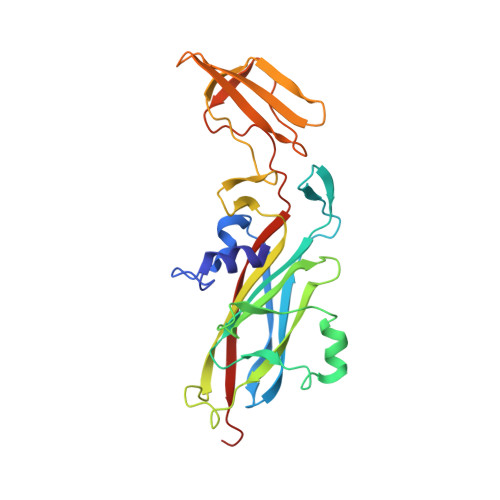
The structure of the complex of plastocyanin and cytochrome f, determined by paramagnetic NMR and restrained rigid-body molecular dynamics.
Ubbink, M., Ejdeback, M., Karlsson, B.G., Bendall, D.S.(1998) Structure 6: 323-335
- PubMed: 9551554
- DOI: https://doi.org/10.1016/s0969-2126(98)00035-5
- Primary Citation of Related Structures:
2PCF - PubMed Abstract:
The reduction of plastocyanin by cytochrome f is part of the chain of photosynthetic electron transfer reactions that links photosystems II and I. The reaction is rapid and is influenced by charged residues on both proteins. Previously determined structures show that the plastocyanin copper and cytochrome f haem redox centres are some distance apart from the relevant charged sidechains, and until now it was unclear how a transient electrostatic complex can be formed that brings the redox centres sufficiently close for a rapid reaction. A new approach was used to determine the structure of the transient complex between cytochrome f and plastocyanin. Diamagnetic chemical shift changes and intermolecular pseudocontact shifts in the NMR spectrum of plastocyanin were used as input in restrained rigid-body molecular dynamics calculations. An ensemble of ten structures was obtained, in which the root mean square deviation of the plastocyanin position relative to cytochrome f is 1.0 A. Electrostatic interaction is maintained at the same time as the hydrophobic side of plastocyanin makes close contact with the haem area, thus providing a short electron transfer pathway (Fe-Cu distance 10.9 A) via residues Tyr1 or Phe4 (cytochrome f) and the copper ligand His87 (plastocyanin). The combined use of diamagnetic and paramagnetic chemical shift changes makes it possible to obtain detailed information about the structure of a transient complex of redox proteins. The structure suggests that the electrostatic interactions 'guide' the partners into a position that is optimal for electron transfer, and which may be stabilised by short-range interactions.
- Department of Biochemistry, University of Cambridge, England. m.ubbink@chem.leidenuniv.nl
Organizational Affiliation: