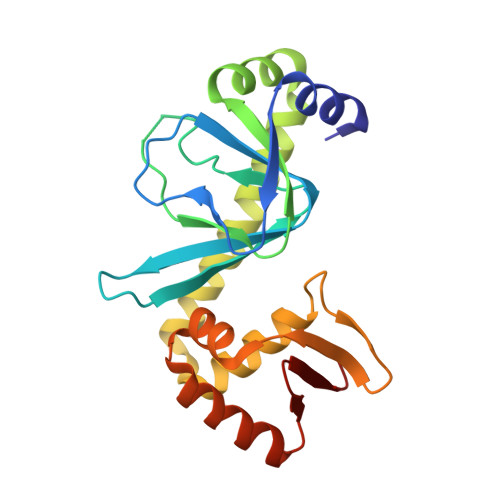
Crystal Structure of the Pseudomonas aeruginosa Virulence Factor Regulator.
Cordes, T.J., Worzalla, G.A., Ginster, A.M., Forest, K.T.(2011) J Bacteriol 193: 4069-4074
- PubMed: 21665969
- DOI: https://doi.org/10.1128/JB.00666-10
- Primary Citation of Related Structures:
2OZ6 - PubMed Abstract:
Virulence factor regulator (Vfr) enhances Pseudomonas aeruginosa pathogenicity through its role as a global transcriptional regulator. The crystal structure of Vfr shows that it is a winged-helix DNA-binding protein like its homologue cyclic AMP receptor protein (CRP). In addition to an expected primary cyclic AMP-binding site, a second ligand-binding site is nestled between the N-terminal domain and the C-terminal helix-turn-helix domain. Unlike CRP, Vfr is a symmetric dimer in the absence of DNA. Removal of seven disordered N-terminal residues of Vfr prevents the growth of P. aeruginosa.
- University of Wisconsin-Madison, Department of Bacteriology, 1550 Linden Dr., Madison, WI 53706, USA.
Organizational Affiliation: