Implications of the structure of a poly-Gln/anti-poly-Gln complex for disease progression and therapy
Li, P.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Fv light chain avriable domain VL | 115 | Mus musculus | Mutation(s): 0 Gene Names: VL | 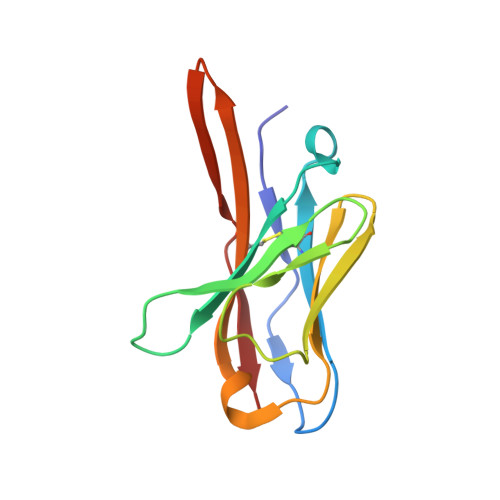 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Fv heavy chain variable domain VH | 118 | Mus musculus | Mutation(s): 0 Gene Names: VH | 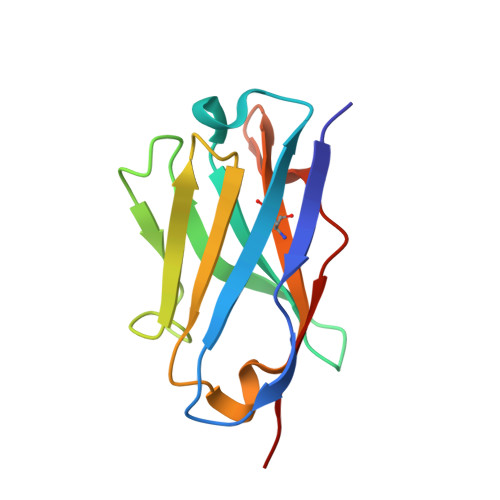 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| poly-Gln peptide antigen | 12 | N/A | Mutation(s): 0 | 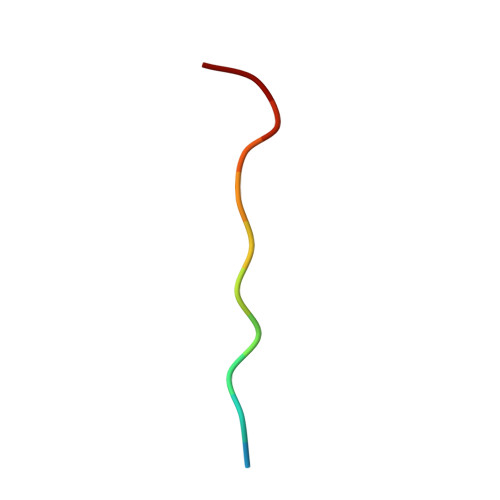 | |
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 165.29 | α = 90 |
| b = 78.724 | β = 90 |
| c = 49.997 | γ = 90 |
| Software Name | Purpose |
|---|---|
| ADSC | data collection |
| CNS | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| CNS | phasing |