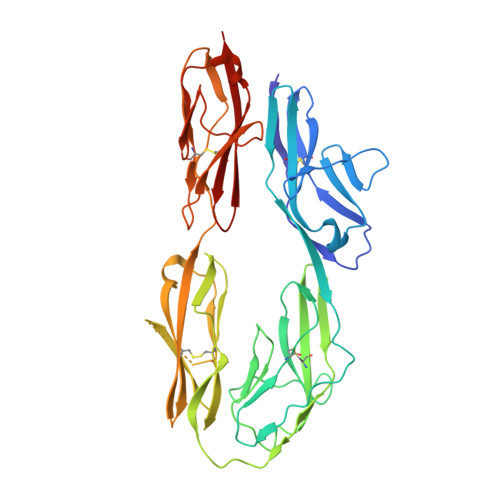
The crystal structure of the ligand-binding module of human TAG-1 suggests a new mode of homophilic interaction
Mortl, M., Sonderegger, P., Diederichs, K., Welte, W.(2007) Protein Sci 16: 2174-2183
- PubMed: 17766378
- DOI: https://doi.org/10.1110/ps.072802707
- Primary Citation of Related Structures:
2OM5 - PubMed Abstract:
Human TAG-1 is a neural cell adhesion molecule that is crucial for the development of the nervous system during embryogenesis. It consists of six immunoglobulin-like and four fibronectin III-like domains and is anchored to the membrane by glycosylphosphatidylinositol. Herein we present the crystal structure of the four N-terminal immunoglobulin-like domains of TAG-1 (TAG-1(Ig1-4)), known to be important in heterophilic and homophilic macromolecular interactions. The contacts of neighboring molecules within the crystal were investigated. A comparison with the structure of the chicken ortholog resulted in an alternative mode for the molecular mechanism of homophilic TAG-1 interaction. This mode of TAG-1 homophilic interaction is based on dimer formation rather than formation of a molecular zipper as proposed for the chicken ortholog.
- University of Konstanz, Department of Biology, Konstanz, Germany.
Organizational Affiliation: