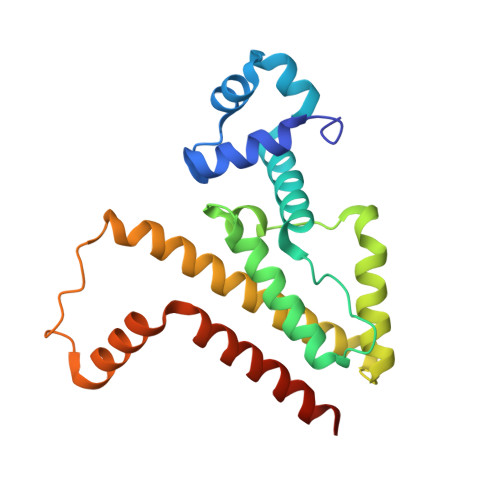
How an agonist peptide mimics the antibiotic tetracycline to induce Tet-repressor
Luckner, S.R., Klotzsche, M., Berens, C., Hillen, W., Muller, Y.A.(2007) J Mol Biology 368: 780-790
- PubMed: 17374541
- DOI: https://doi.org/10.1016/j.jmb.2007.02.030
- Primary Citation of Related Structures:
2NS7, 2NS8 - PubMed Abstract:
A 16-residue peptide, called Tip, induces the tetracycline repressor TetR as efficiently as the antibiotic tetracycline when fused to the N or C terminus of another protein. This is unusual because the majority of in vitro selected peptides, such as Tip, inhibit protein function, and agonist peptides are only rarely identified. We elucidated the atomic mechanism of TetR induction by Tip from crystal structures of TetR in complex with Tip and of free TetR. Peptide induction ultimately results in the same movements of DNA reading heads, but Tip accomplishes this by very different molecular interactions from tetracycline involving important contacts to the TetR surface. As a direct consequence, an alternate pathway of allostery becomes possible that makes ample use of intersubunit interactions. For the first time it is possible to show in atomic detail how a small molecule controlled bacterial transcription factor such as TetR becomes responsive to protein-protein interactions, characteristic of gene transcription regulation in higher organisms.
- Lehrstuhl für Biotechnik, Institut für Biologie, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany.
Organizational Affiliation: