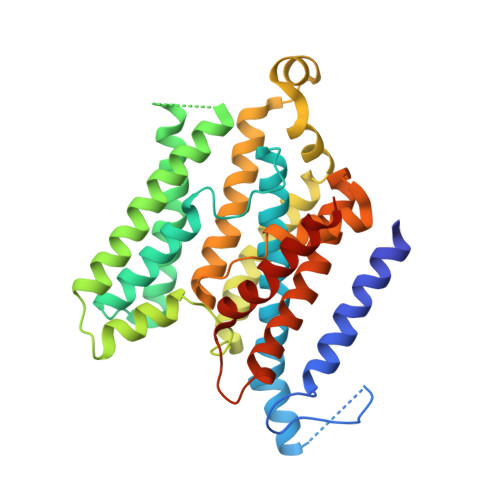
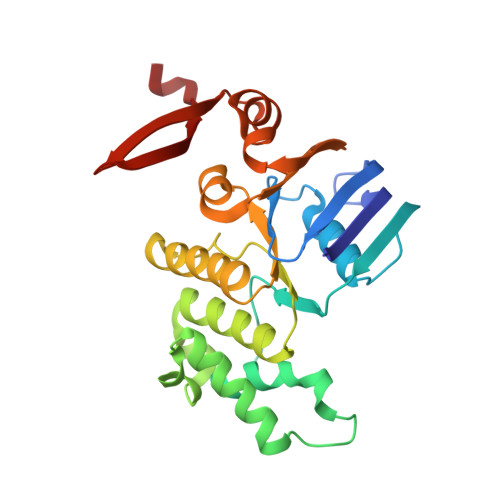
An inward-facing conformation of a putative metal-chelate-type ABC transporter.
Pinkett, H.W., Lee, A.T., Lum, P., Locher, K.P., Rees, D.C.(2007) Science 315: 373-377
- PubMed: 17158291
- DOI: https://doi.org/10.1126/science.1133488
- Primary Citation of Related Structures:
2NQ2 - PubMed Abstract:
The crystal structure of a putative metal-chelate-type adenosine triphosphate (ATP)-binding cassette (ABC) transporter encoded by genes HI1470 and HI1471 of Haemophilus influenzae has been solved at 2.4 angstrom resolution. The permeation pathway exhibits an inward-facing conformation, in contrast to the outward-facing state previously observed for the homologous vitamin B12 importer BtuCD. Although the structures of both HI1470/1 and BtuCD have been solved in nucleotide-free states, the pairs of ABC subunits in these two structures differ by a translational shift in the plane of the membrane that coincides with a repositioning of the membrane-spanning subunits. The differences observed between these ABC transporters involve relatively modest rearrangements and may serve as structural models for inward- and outward-facing conformations relevant to the alternating access mechanism of substrate translocation.
- Division of Chemistry and Chemical Engineering, Howard Hughes Medical Institute, MC 114-96, California Institute of Technology (Caltech), Pasadena, CA 91125, USA.
Organizational Affiliation: