The Q403K mutant heme domain of flavocytochrome P450 BM3
Clark, J.P., Anderson, J.L.R., Miles, C.S., Mowat, C.G., Reid, G.A., Chapman, S.K.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Bifunctional P-450:NADPH-P450 reductase | 471 | Priestia megaterium | Mutation(s): 1 Gene Names: Cyp102a21 EC: 1.14.14.1 (PDB Primary Data), 1.6.2.4 (UniProt) | 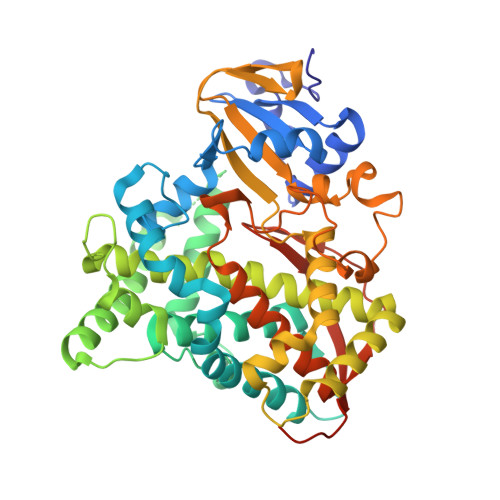 | |
UniProt | |||||
Find proteins for P14779 (Priestia megaterium (strain ATCC 14581 / DSM 32 / CCUG 1817 / JCM 2506 / NBRC 15308 / NCIMB 9376 / NCTC 10342 / NRRL B-14308 / VKM B-512 / Ford 19)) Explore P14779 Go to UniProtKB: P14779 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P14779 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| HEM Query on HEM | C [auth A], D [auth B] | PROTOPORPHYRIN IX CONTAINING FE C34 H32 Fe N4 O4 KABFMIBPWCXCRK-RGGAHWMASA-L |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 58.753 | α = 90 |
| b = 152.792 | β = 94.67 |
| c = 61.504 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| ADSC | data collection |
| HKL-2000 | data reduction |
| SCALEPACK | data scaling |
| AMoRE | phasing |