Pro-sfcapsase-1, structural insights into activation mechanism of caspases
Ni, L., Yu, Z., Lemongello, D., Friesen, P.D., Fisher, A.J.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Caspase-1 | A [auth C], B [auth D] | 310 | Spodoptera frugiperda | Mutation(s): 1 EC: 3.4.22.36 (PDB Primary Data), 3.4.22 (UniProt) | 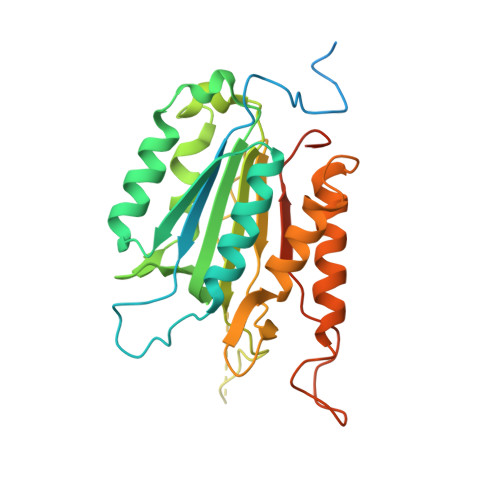 |
UniProt | |||||
Find proteins for P89116 (Spodoptera frugiperda) Explore P89116 Go to UniProtKB: P89116 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P89116 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 106.223 | α = 90 |
| b = 106.223 | β = 90 |
| c = 113.595 | γ = 120 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| HKL-2000 | data collection |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| AMoRE | phasing |