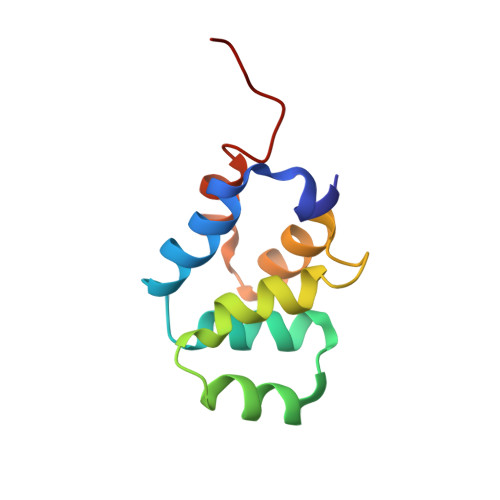
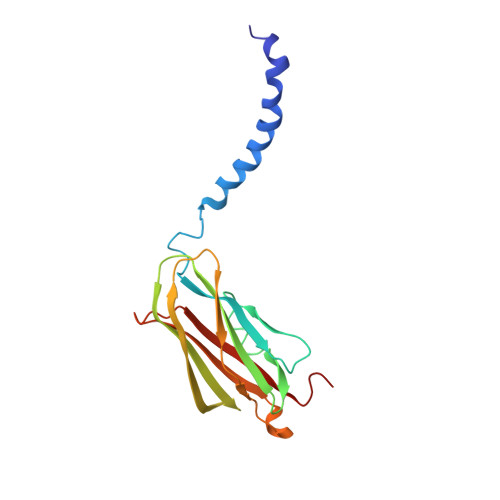
Structural basis of death domain signaling in the p75 neurotrophin receptor
Lin, Z., Tann, J.Y., Goh, E.T., Kelly, C., Lim, K.B., Gao, J.F., Ibanez, C.F.(2015) Elife 4: 11692-11692
- PubMed: 26646181
- DOI: https://doi.org/10.7554/eLife.11692
- Primary Citation of Related Structures:
2N7Z, 2N80, 2N83, 2N97 - PubMed Abstract:
Death domains (DDs) mediate assembly of oligomeric complexes for activation of downstream signaling pathways through incompletely understood mechanisms. Here we report structures of complexes formed by the DD of p75 neurotrophin receptor (p75(NTR)) with RhoGDI, for activation of the RhoA pathway, with caspase recruitment domain (CARD) of RIP2 kinase, for activation of the NF-kB pathway, and with itself, revealing how DD dimerization controls access of intracellular effectors to the receptor. RIP2 CARD and RhoGDI bind to p75(NTR) DD at partially overlapping epitopes with over 100-fold difference in affinity, revealing the mechanism by which RIP2 recruitment displaces RhoGDI upon ligand binding. The p75(NTR) DD forms non-covalent, low-affinity symmetric dimers in solution. The dimer interface overlaps with RIP2 CARD but not RhoGDI binding sites, supporting a model of receptor activation triggered by separation of DDs. These structures reveal how competitive protein-protein interactions orchestrate the hierarchical activation of downstream pathways in non-catalytic receptors.
- Department of Physiology, National University of Singapore, Singapore, Singapore.
Organizational Affiliation: