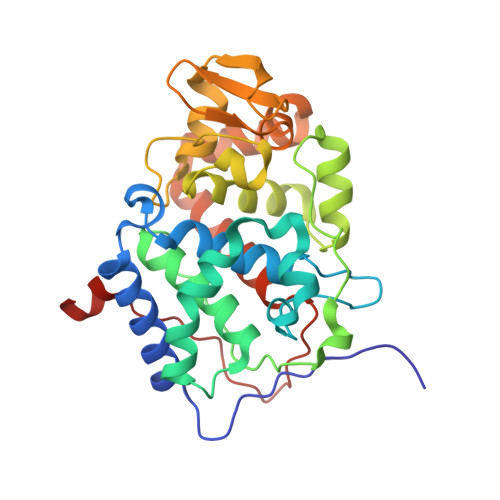
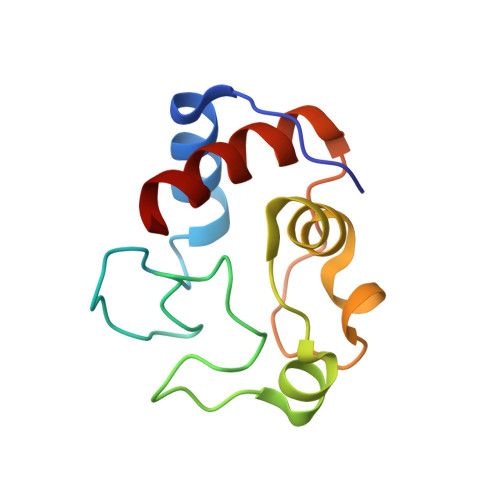
The low-affinity complex of cytochrome c and its peroxidase.
Van de Water, K., Sterckx, Y.G., Volkov, A.N.(2015) Nat Commun 6: 7073-7073
- PubMed: 25944250
- DOI: https://doi.org/10.1038/ncomms8073
- Primary Citation of Related Structures:
2N18 - PubMed Abstract:
The complex of yeast cytochrome c peroxidase and cytochrome c is a paradigm of the biological electron transfer (ET). Building on seven decades of research, two different models have been proposed to explain its functional redox activity. One postulates that the intermolecular ET occurs only in the dominant, high-affinity protein-protein orientation, while the other posits formation of an additional, low-affinity complex, which is much more active than the dominant one. Unlike the high-affinity interaction-extensively studied by X-ray crystallography and NMR spectroscopy-until now the binding of cytochrome c to the low-affinity site has not been observed directly, but inferred mainly from kinetics experiments. Here we report the structure of this elusive, weak protein complex and show that it consists of a dominant, inactive bound species and an ensemble of minor, ET-competent protein-protein orientations, which summarily account for the experimentally determined value of the ET rate constant.
- 1] Jean Jeener NMR Centre, Structural Biology Brussels, Vrije Universiteit Brussel, Pleinlaan 2, Brussels 1050, Belgium [2] Structural Biology Research Center, VIB, Pleinlaan 2, Brussels 1050, Belgium.
Organizational Affiliation: