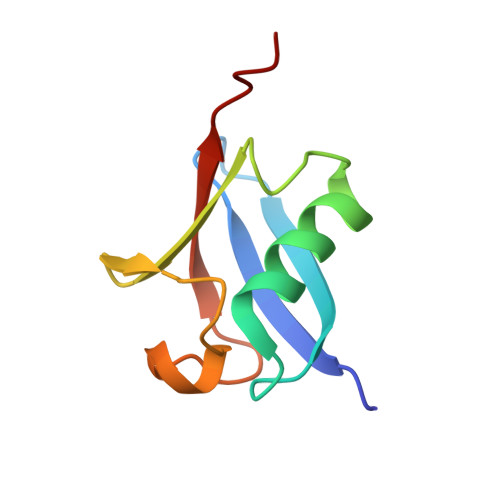
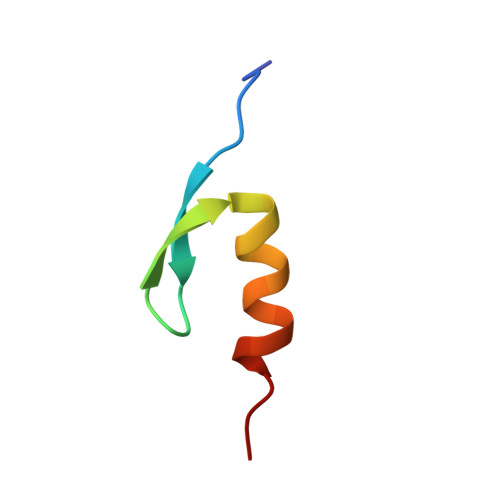
NMR Structure of the Human Rad18 Zinc Finger in Complex with Ubiquitin Defines a Class of UBZ Domains in Proteins Linked to the DNA Damage Response.
Rizzo, A.A., Salerno, P.E., Bezsonova, I., Korzhnev, D.M.(2014) Biochemistry 53: 5895-5906
- PubMed: 25162118
- DOI: https://doi.org/10.1021/bi500823h
- Primary Citation of Related Structures:
2MRE, 2MRF - PubMed Abstract:
Ubiquitin-mediated interactions are critical for the cellular DNA damage response (DDR). Therefore, many DDR-related proteins contain ubiquitin-binding domains, including ubiquitin-binding zinc fingers (UBZs). The majority of these UBZ domains belong to the C2H2 (type 3 Polη-like) or C2HC (type 4 Rad18-like) family. We have used nuclear magnetic resonance (NMR) spectroscopy to characterize the binding to ubiquitin and determine the structure of the type 4 UBZ domain (UBZ4) from human Rad18, which is a key ubiquitin ligase in the DNA damage tolerance pathway responsible for monoubiquitination of the DNA sliding clamp PCNA. The Rad18-UBZ domain binds ubiquitin with micromolar affinity and adopts a β1-β2-α fold similar to the previously characterized type 3 UBZ domain (UBZ3) from the translesion synthesis DNA polymerase Polη. However, despite nearly identical structures, a disparity in the location of binding-induced NMR chemical shift perturbations shows that the Rad18-UBZ4 and Polη-UBZ3 domains bind ubiquitin in distinctly different modes. The Rad18-UBZ4 domain interacts with ubiquitin with the α-helix and strand β1 as shown by the structure of the Rad18-UBZ domain-ubiquitin complex determined in this work, while the Polη-UBZ3 domain exclusively utilizes the α-helix. Our findings suggest the existence of two classes of UBZ domains in DDR-related proteins with similar structures but unique ubiquitin binding properties and provide context for further study to establish the differential roles of these domains in the complex cellular response to DNA damage.
- Department of Molecular Biology and Biophysics, University of Connecticut Health Center , Farmington, Connecticut 06030, United States.
Organizational Affiliation: