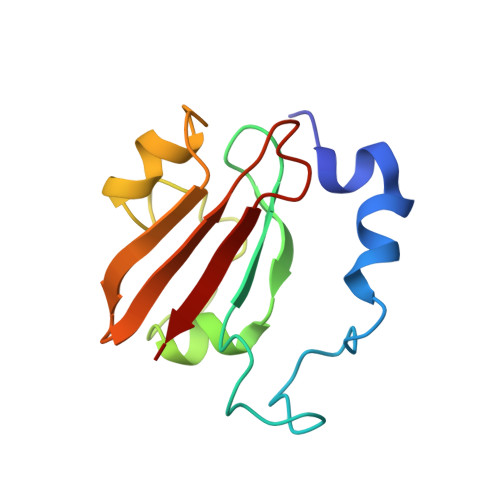
Solution Structure of the PAS Domain of a Thermophilic YybT Protein Homolog Reveals a Potential Ligand-binding Site.
Tan, E., Rao, F., Pasunooti, S., Pham, T.H., Soehano, I., Turner, M.S., Liew, C.W., Lescar, J., Pervushin, K., Liang, Z.X.(2013) J Biological Chem 288: 11949-11959
- PubMed: 23504327
- DOI: https://doi.org/10.1074/jbc.M112.437764
- Primary Citation of Related Structures:
2M1C - PubMed Abstract:
The Bacillus subtilis protein YybT (or GdpP) and its homologs were recently established as stress signaling proteins that exert their biological effect by degrading the bacterial messenger cyclic di-AMP. YybT homologs contain a small Per-ARNT-Sim (PAS) domain (~80 amino acids) that can bind b-type heme with 1:1 stoichiometry despite the small size of the domain and the lack of a conserved heme iron-coordinating residue. We determined the solution structure of the PAS domain of GtYybT from Geobacillus thermodenitrificans by NMR spectroscopy to further probe its function. The solution structure confirms that PASGtYybT adopts the characteristic PAS fold composed of a five-stranded antiparallel β sheet and a few short α-helices. One α-helix and three central β-strands of PASGtYybT are noticeably shorter than those of the typical PAS domains. Despite the small size of the protein domain, a hydrophobic pocket is formed by the side chains of nonpolar residues stemming from the β-strands and α-helices. A set of residues in the vicinity of the pocket and in the C-terminal region at the dimeric interface exhibits perturbed NMR parameters in the presence of heme or zinc protoporphyrin. Together, the results unveil a compact PAS domain with a potential ligand-binding pocket and reinforce the view that the PASYybT domains function as regulatory domains in the modulation of cellular cyclic di-AMP concentration.
- Division of Structural Biology and Biochemistry, School of Biological Sciences, Nanyang Technological University, 60 Nanyang Drive, Singapore 637551.
Organizational Affiliation: