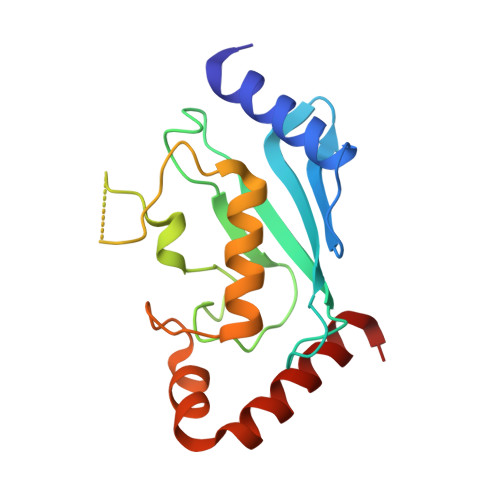
Allosteric regulation of E2:E3 interactions promote a processive ubiquitination machine.
Das, R., Liang, Y.H., Mariano, J., Li, J., Huang, T., King, A., Tarasov, S.G., Weissman, A.M., Ji, X., Byrd, R.A.(2013) EMBO J 32: 2504-2516
- PubMed: 23942235
- DOI: https://doi.org/10.1038/emboj.2013.174
- Primary Citation of Related Structures:
2LXH, 2LXP, 4LAD - PubMed Abstract:
RING finger proteins constitute the large majority of ubiquitin ligases (E3s) and function by interacting with ubiquitin-conjugating enzymes (E2s) charged with ubiquitin. How low-affinity RING-E2 interactions result in highly processive substrate ubiquitination is largely unknown. The RING E3, gp78, represents an excellent model to study this process. gp78 includes a high-affinity secondary binding region for its cognate E2, Ube2g2, the G2BR. The G2BR allosterically enhances RING:Ube2g2 binding and ubiquitination. Structural analysis of the RING:Ube2g2:G2BR complex reveals that a G2BR-induced conformational effect at the RING:Ube2g2 interface is necessary for enhanced binding of RING to Ube2g2 or Ube2g2 conjugated to Ub. This conformational effect and a key ternary interaction with conjugated ubiquitin are required for ubiquitin transfer. Moreover, RING:Ube2g2 binding induces a second allosteric effect, disrupting Ube2g2:G2BR contacts, decreasing affinity and facilitating E2 exchange. Thus, gp78 is a ubiquitination machine where multiple E2-binding sites coordinately facilitate processive ubiquitination.
- Structural Biophysics Laboratory, Center for Cancer Research, National Cancer Institute, Frederick, MD, USA.
Organizational Affiliation: