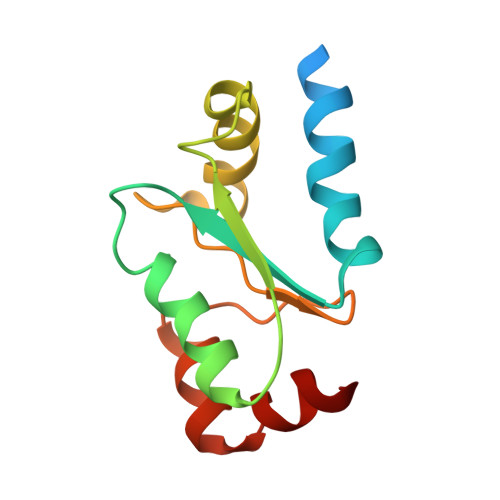
Structural analysis of glutaredoxin domain of Mus musculus thioredoxin glutathione reductase
Dobrovolska, O., Shumilina, E., Gladyshev, V.N., Dikiy, A.(2012) PLoS One 7: e52914-e52914
- PubMed: 23300818
- DOI: https://doi.org/10.1371/journal.pone.0052914
- Primary Citation of Related Structures:
2LV3 - PubMed Abstract:
Thioredoxin glutathione reductase (TGR) is a member of the mammalian thioredoxin reductase family that has a monothiol glutaredoxin (Grx) domain attached to the thioredoxin reductase module. Here, we report a structure of the Grx domain of mouse TGR, determined through high resolution NMR spectroscopy to the final backbone RMSD value of 0.48 ± 0.10 Å. The structure represents a sandwich-like molecule composed of a four stranded β-sheet flanked by five α-helixes, with the CxxS active motif located on the catalytic loop. We structurally characterized the glutathione-binding site in the protein and describe sequence and structural relationships of the domain with glutaredoxins. The structure illuminates a key functional center that evolved in mammalian TGRs to act in thiol-disulfide reactions. Our study allows us to hypothesize that Cys105 might be functionally relevant for TGR catalysis. In addition, the data suggest that the N-terminus of Grx acts as a possible regulatory signal also protecting the protein active site from unwanted interactions in cellular cytosol.
- Department of Biotechnology, Norwegian University of Science and Technology, Trondheim, Norway.
Organizational Affiliation: