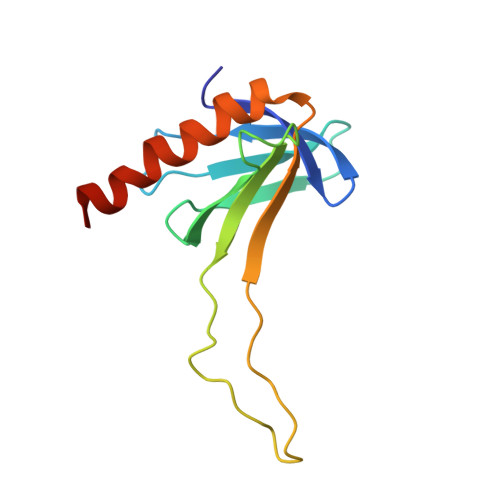
Structural and functional characterization of interactions involving the Tfb1 subunit of TFIIH and the NER factor Rad2.
Lafrance-Vanasse, J., Arseneault, G., Cappadocia, L., Chen, H.T., Legault, P., Omichinski, J.G.(2012) Nucleic Acids Res 40: 5739-5750
- PubMed: 22373916
- DOI: https://doi.org/10.1093/nar/gks194
- Primary Citation of Related Structures:
2LOX - PubMed Abstract:
The general transcription factor IIH (TFIIH) plays crucial roles in transcription as part of the pre-initiation complex (PIC) and in DNA repair as part of the nucleotide excision repair (NER) machinery. During NER, TFIIH recruits the 3'-endonuclease Rad2 to damaged DNA. In this manuscript, we functionally and structurally characterized the interaction between the Tfb1 subunit of TFIIH and Rad2. We show that deletion of either the PH domain of Tfb1 (Tfb1PH) or several segments of the Rad2 spacer region yield yeast with enhanced sensitivity to UV irradiation. Isothermal titration calorimetry studies demonstrate that two acidic segments of the Rad2 spacer bind to Tfb1PH with nanomolar affinity. Structure determination of a Rad2-Tfb1PH complex indicates that Rad2 binds to TFIIH using a similar motif as TFIIEα uses to bind TFIIH in the PIC. Together, these results provide a mechanistic bridge between the role of TFIIH in transcription and DNA repair.
- Département de Biochimie, Université de Montréal, C.P. 6128, Succursale Centre-Ville, Montréal, QC H3C 3J7, Canada.
Organizational Affiliation: