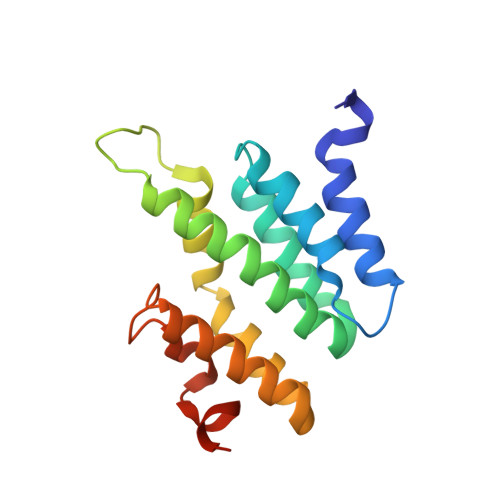
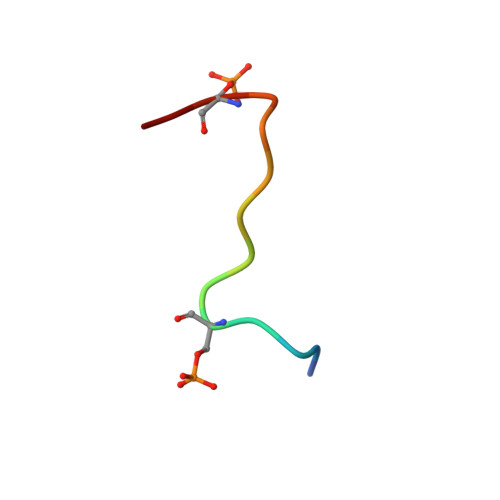
Serine phosphorylation and proline isomerization in RNAP II CTD control recruitment of Nrd1.
Kubicek, K., Cerna, H., Holub, P., Pasulka, J., Hrossova, D., Loehr, F., Hofr, C., Vanacova, S., Stefl, R.(2012) Genes Dev 26: 1891-1896
- PubMed: 22892239
- DOI: https://doi.org/10.1101/gad.192781.112
- Primary Citation of Related Structures:
2LO6 - PubMed Abstract:
Recruitment of appropriate RNA processing factors to the site of transcription is controlled by post-translational modifications of the C-terminal domain (CTD) of RNA polymerase II (RNAP II). Here, we report the solution structure of the Ser5 phosphorylated (pSer5) CTD bound to Nrd1. The structure reveals a direct recognition of pSer5 by Nrd1 that requires the cis conformation of the upstream pSer5-Pro6 peptidyl-prolyl bond of the CTD. Mutations at the complex interface diminish binding affinity and impair processing or degradation of noncoding RNAs. These findings underpin the interplay between covalent and noncovalent changes in the CTD structure that constitute the CTD code.
- CEITEC-Central European Institute of Technology, Masaryk University, Brno, 62500, Czech Republic.
Organizational Affiliation: