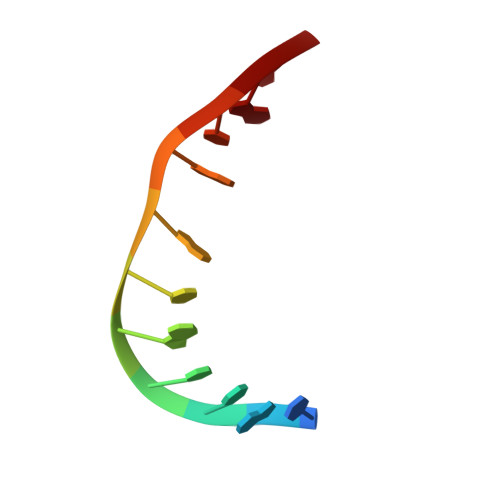
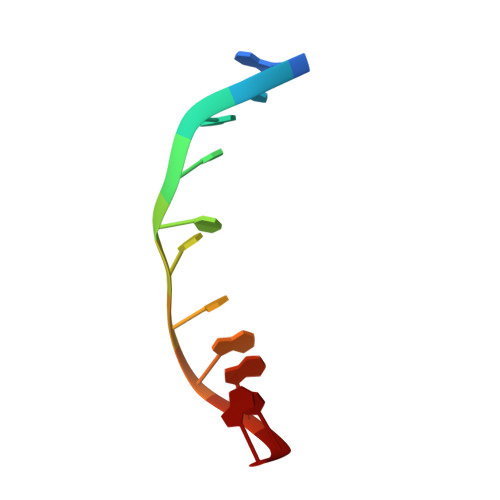
Double threading through DNA: NMR structural study of a bis-naphthalene macrocycle bound to a thymine-thymine mismatch.
Jourdan, M., Granzhan, A., Guillot, R., Dumy, P., Teulade-Fichou, M.P.(2012) Nucleic Acids Res 40: 5115-5128
- PubMed: 22362757
- DOI: https://doi.org/10.1093/nar/gks067
- Primary Citation of Related Structures:
2LL9, 2LLJ - PubMed Abstract:
The macrocyclic bis-naphthalene macrocycle (2,7-BisNP), belonging to the cyclobisintercalator family of DNA ligands, recognizes T-T mismatch sites in duplex DNA with high affinity and selectivity, as evidenced by thermal denaturation experiments and NMR titrations. The binding of this macrocycle to an 11-mer DNA oligonucleotide containing a T-T mismatch was studied using NMR spectroscopy and NMR-restrained molecular modeling. The ligand forms a single type of complex with the DNA, in which one of the naphthalene rings of the ligand occupies the place of one of the mismatched thymines, which is flipped out of the duplex. The second naphthalene unit of the ligand intercalates at the A-T base pair flanking the mismatch site, leading to encapsulation of its thymine residue via double stacking. The polyammonium linking chains of the macrocycle are located in the minor and the major grooves of the oligonucleotide and participate in the stabilization of the complex by formation of hydrogen bonds with the encapsulated thymine base and the mismatched thymine remaining inside the helix. The study highlights the uniqueness of this cyclobisintercalation binding mode and its importance for recognition of DNA lesion sites by small molecules.
- CNRS UMR5250, ICMG FR2607, Département de Chimie Moléculaire, Université Joseph Fourier, 570 rue de la Chimie, 38041 Grenoble Cedex 9, France. muriel.jourdan@ujf-grenoble.fr
Organizational Affiliation: