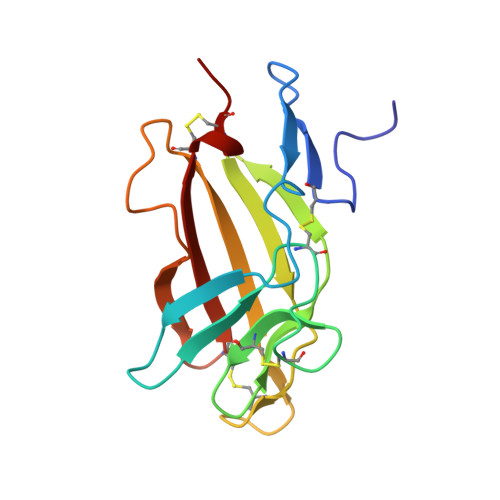
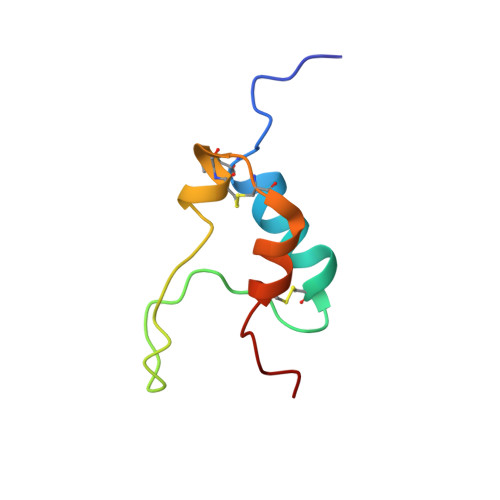
An exon splice enhancer primes IGF2:IGF2R binding site structure and function evolution.
Williams, C., Hoppe, H.J., Rezgui, D., Strickland, M., Forbes, B.E., Grutzner, F., Frago, S., Ellis, R.Z., Wattana-Amorn, P., Prince, S.N., Zaccheo, O.J., Nolan, C.M., Mungall, A.J., Jones, E.Y., Crump, M.P., Hassan, A.B.(2012) Science 338: 1209-1213
- PubMed: 23197533
- DOI: https://doi.org/10.1126/science.1228633
- Primary Citation of Related Structures:
2L29, 2L2A, 2L2G, 2LLA - PubMed Abstract:
Placental development and genomic imprinting coevolved with parental conflict over resource distribution to mammalian offspring. The imprinted genes IGF2 and IGF2R code for the growth promoter insulin-like growth factor 2 (IGF2) and its inhibitor, mannose 6-phosphate (M6P)/IGF2 receptor (IGF2R), respectively. M6P/IGF2R of birds and fish do not recognize IGF2. In monotremes, which lack imprinting, IGF2 specifically bound M6P/IGF2R via a hydrophobic CD loop. We show that the DNA coding the CD loop in monotremes functions as an exon splice enhancer (ESE) and that structural evolution of binding site loops (AB, HI, FG) improved therian IGF2 affinity. We propose that ESE evolution led to the fortuitous acquisition of IGF2 binding by M6P/IGF2R that drew IGF2R into parental conflict; subsequent imprinting may then have accelerated affinity maturation.
- Department of Organic and Biological Chemistry, School of Chemistry, University of Bristol, Bristol BS8 1TS, UK.
Organizational Affiliation: