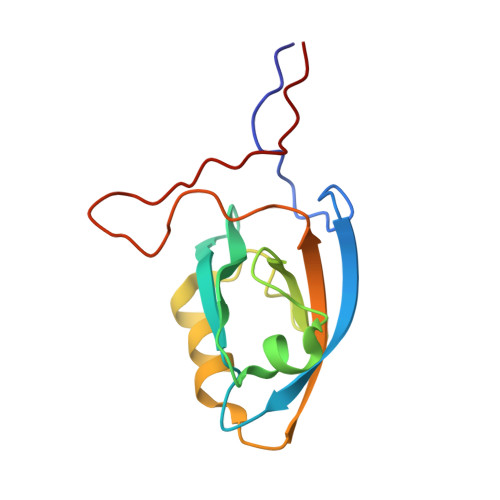
The structural and dynamic response of MAGI-1 PDZ1 with non-canonical domain boundaries to binding of human papillomavirus (HPV) E6
Charbonnier, S., Nomine, Y., Ramirez, J., Luck, K., Chapelle, A., Stote, R.H., Trave, G., Kieffer, B., Atkinson, R.A.(2011) J Mol Biology
- PubMed: 21238461
- DOI: https://doi.org/10.1016/j.jmb.2011.01.015
- Primary Citation of Related Structures:
2KPK, 2KPL - PubMed Abstract:
PDZ domains are protein interaction domains that are found in cytoplasmic proteins involved in signaling pathways and subcellular transport. Their roles in the control of cell growth, cell polarity, and cell adhesion in response to cell contact render this family of proteins targets during the development of cancer. Targeting of these network hubs by the oncoprotein E6 of "high-risk" human papillomaviruses (HPVs) serves to effect the efficient disruption of cellular processes. Using NMR, we have solved the three-dimensional solution structure of an extended construct of the second PDZ domain of MAGI-1 (MAGI-1 PDZ1) alone and bound to a peptide derived from the C-terminus of HPV16 E6, and we have characterized the changes in backbone dynamics and hydrogen bonding that occur upon binding. The binding event induces quenching of high-frequency motions in the C-terminal tail of the PDZ domain, which contacts the peptide upstream of the canonical X-[T/S]-X-[L/V] binding motif. Mutations designed in the C-terminal flanking region of the PDZ domain resulted in a significant decrease in binding affinity for E6 peptides. This detailed analysis supports the notion of a global response of the PDZ domain to the binding event, with effects propagated to distal sites, and reveals unexpected roles for the sequences flanking the canonical PDZ domain boundaries.
- Equipe Oncoprotéines, Ecole Supérieure de Biotechnologie de Strasbourg, Boulevard Sébastien Brant, BP 10413, 67412 Illkirch Cedex, France.
Organizational Affiliation: