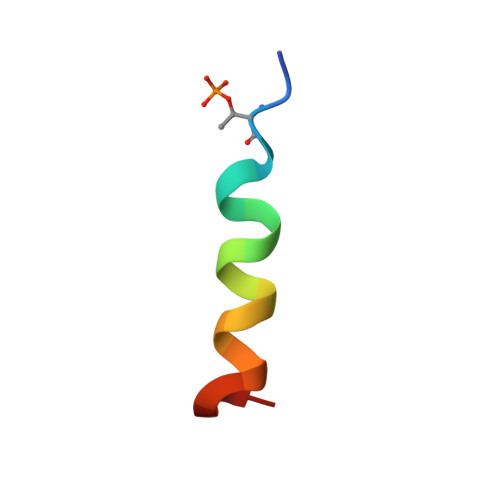
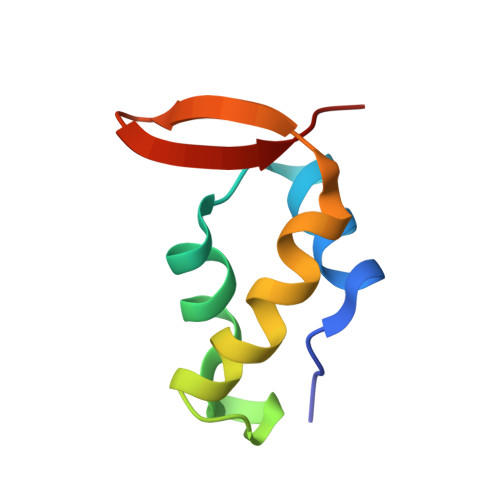
NMR structure of a complex formed by the carboxyl-terminal domain of human RAP74 and a phosphorylated peptide from the central domain of the FCP1 phosphatase
Yang, A., Abbott, K.L., Desjardins, A., Di Lello, P., Omichinski, J.G., Legault, P.(2009) Biochemistry 48: 1964-1974
- PubMed: 19215094
- DOI: https://doi.org/10.1021/bi801549m
- Primary Citation of Related Structures:
2K7L - PubMed Abstract:
Recycling of RNA polymerase II (RNAPII) requires dephosphorylation of the C-terminal domain (CTD) of the largest subunit of the polymerase. FCP1 enables the recycling of RNAPII via its CTD-specific phosphatase activity, which is stimulated by the RAP74 subunit of the general transcription factor TFIIF. Both the central (centFCP1) and C-terminal (cterFCP1) domains of FCP1 interact independently and specifically with the C-terminal domain of RAP74 (cterRAP74), suggesting that these interactions mediate the stimulatory effect of TFIIF on the CTD phosphatase activity of FCP1. Phosphorylation of FCP1 by casein kinase 2 on residues in its central (T584) and C-terminal (S942 and S944) domains stimulates its binding to RAP74 and its CTD phosphatase activity. To improve our understanding of the FCP1-RAP74 interactions, we previously determined the NMR structure of a complex formed by human cterRAP74 and cterFCP1. We now present the high-resolution NMR structure and thermodynamic characterization by isothermal titration calorimetry of a complex formed by the same cterRAP74 domain and a phosphorylated peptide from the central domain of human FCP1 (centFCP1-PO(4)). Comparison of the cterFCP1-cterRAP74 and centFCP1-PO(4)-cterRAP74 complexes indicates that centFCP1 and cterFCP1 both utilize hydrophobic and acidic residues to recognize the same groove of RAP74, but there are significant differences in the details of their interactions. These differences point to the adaptability of RAP74 to recognize the two regions of FCP1. Our NMR and thermodynamic studies further elucidate the complex molecular mechanism by which TFIIF and FCP1 cooperate for RNAPII recycling.
- Département de Biochimie, Université de Montréal, Succursale Centre-Ville, QC, Canada.
Organizational Affiliation: