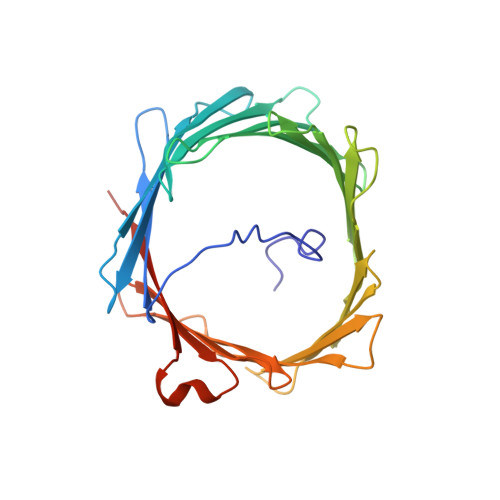
Solution structure of the integral human membrane protein VDAC-1 in detergent micelles.
Hiller, S., Garces, R.G., Malia, T.J., Orekhov, V.Y., Colombini, M., Wagner, G.(2008) Science 321: 1206-1210
- PubMed: 18755977
- DOI: https://doi.org/10.1126/science.1161302
- Primary Citation of Related Structures:
2K4T - PubMed Abstract:
The voltage-dependent anion channel (VDAC) mediates trafficking of small molecules and ions across the eukaryotic outer mitochondrial membrane. VDAC also interacts with antiapoptotic proteins from the Bcl-2 family, and this interaction inhibits release of apoptogenic proteins from the mitochondrion. We present the nuclear magnetic resonance (NMR) solution structure of recombinant human VDAC-1 reconstituted in detergent micelles. It forms a 19-stranded beta barrel with the first and last strand parallel. The hydrophobic outside perimeter of the barrel is covered by detergent molecules in a beltlike fashion. In the presence of cholesterol, recombinant VDAC-1 can form voltage-gated channels in phospholipid bilayers similar to those of the native protein. NMR measurements revealed the binding sites of VDAC-1 for the Bcl-2 protein Bcl-x(L), for reduced beta-nicotinamide adenine dinucleotide, and for cholesterol. Bcl-x(L) interacts with the VDAC barrel laterally at strands 17 and 18.
- Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, MA 02115, USA.
Organizational Affiliation: