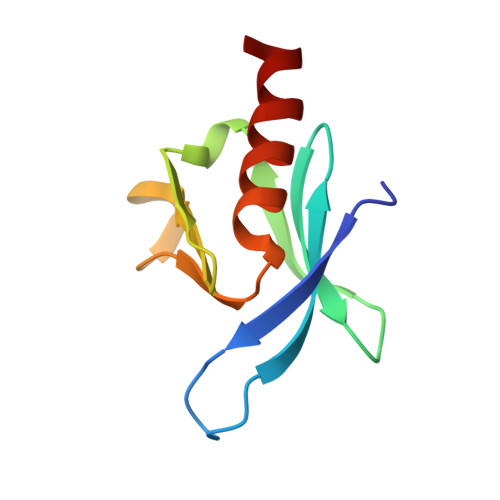
Structural basis for the interaction between the cytoplasmic domain of the hyaluronate receptor layilin and the talin F3 subdomain
Wegener, K.L., Basran, J., Bagshaw, C.R., Campbell, I.D., Roberts, G.C., Critchley, D.R., Barsukov, I.L.(2008) J Mol Biology 382: 112-126
- PubMed: 18638481
- DOI: https://doi.org/10.1016/j.jmb.2008.06.087
- Primary Citation of Related Structures:
2K00 - PubMed Abstract:
Talin is a large cytoskeletal protein that is involved in coupling the integrin family of cell adhesion molecules to the actin cytoskeleton, colocalising with the integrins in focal adhesions (FAs). However, at the leading edge of motile cells, talin colocalises with the hyaluronan receptor layilin in what are thought to be transient adhesions, some of which subsequently mature into more stable FAs. During this maturation process, layilin is replaced with integrins, which are highly clustered in FAs, where localised production of PI(4,5)P(2) by type 1 phosphatidyl inositol phosphate kinase type 1gamma (PIPK1gamma) is thought to play a role in FA assembly. The talin FERM F3 subdomain binds both the integrin beta-subunit cytoplasmic domain and PIPK1gamma, and these interactions are understood in detail at the atomic level. The talin F3 domain also binds to short sequences in the layilin cytoplasmic domain, and here we report the structure of the talin/layilin complex, which shows that talin binds integrins, PIPK1gamma and layilin in similar although subtly different ways. Based on structure comparisons, we designed a set of talin F3 mutations that selectively affected the affinity of talin for its targets, as determined by stopped-flow fluorescence measurements. Such mutations will help to assess the importance of the interactions between talin and its various ligands in cell adhesion and migration.
- Department of Biochemistry, University of Oxford, Oxford OX1 3QU, UK.
Organizational Affiliation: