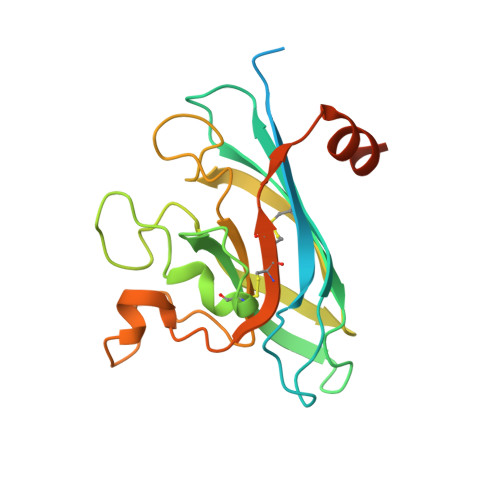
The Structure of Human Extracellular Copper-Zinc Superoxide Dismutase at 1.7 A Resolution: Insights Into Heparin and Collagen Binding.
Antonyuk, S.V., Strange, R.W., Marklund, S.L., Hasnain, S.S.(2009) J Mol Biology 388: 310
- PubMed: 19289127
- DOI: https://doi.org/10.1016/j.jmb.2009.03.026
- Primary Citation of Related Structures:
2JLP - PubMed Abstract:
Extracellular superoxide dismutase (SOD3) is a homotetrameric copper- and zinc-containing glycoprotein with affinity for heparin. The level of SOD3 is particularly high in blood vessel walls and in the lungs. The enzyme has multiple roles including protection of the lungs against hyperoxia and preservation of nitric oxide. The common mutation R213G, which reduces the heparin affinity of SOD3, is associated with increased risk of myocardial infarctions and stroke. We report the first crystal structure of human SOD3 at 1.7 A resolution. The overall subunit fold and the subunit-subunit interface of the SOD3 dimer are similar to the corresponding structures in Cu-Zn SOD (SOD1). The metal-binding sites are similar to those found in SOD1, but with Asn180 replacing Thr137 at the Cu-binding site and a much shorter loop at the zinc-binding site. The dimers form a functional homotetramer that is fashioned through contacts between two extended loops on each subunit. The N- and C-terminal end regions required for tetramerisation and heparin binding, respectively, are highly flexible. Two grooves fashioned by the tetramer interface are suggestive as the probable sites for heparin and collagen binding.
- Molecular Biophysics Group, School of Biological Sciences, University of Liverpool, Liverpool L69 7ZB, UK.
Organizational Affiliation: