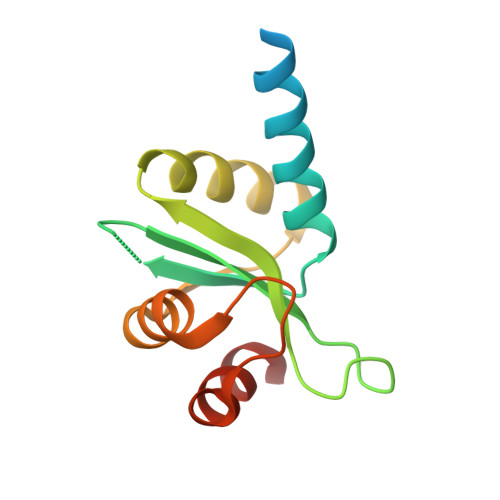
Atomic Resolution Structure of the Cytoplasmic Domain of Yersinia Pestis Yscu, a Regulatory Switch Involved in Type III Secretion.
Lountos, G.T., Austin, B.P., Nallamsetty, S., Waugh, D.S.(2009) Protein Sci 18: 467
- PubMed: 19165725
- DOI: https://doi.org/10.1002/pro.56
- Primary Citation of Related Structures:
2JLH, 2JLI, 2JLJ - PubMed Abstract:
Crystal structures of cleaved and uncleaved forms of the YscU cytoplasmic domain, an essential component of the type III secretion system (T3SS) in Yersinia pestis, have been solved by single-wavelength anomolous dispersion and refined with X-ray diffraction data extending up to atomic resolution (1.13 A). These crystallographic studies provide structural insights into the conformational changes induced upon auto-cleavage of the cytoplasmic domain of YscU. The structures indicate that the cleaved fragments remain bound to each other. The conserved NPTH sequence that contains the site of the N263-P264 peptide bond cleavage is found on a beta-turn which, upon cleavage, undergoes a major reorientation of the loop away from the catalytic N263, resulting in altered electrostatic surface features at the site of cleavage. Additionally, a significant conformational change was observed in the N-terminal linker regions of the cleaved and noncleaved forms of YscU which may correspond to the molecular switch that influences substrate specificity. The YscU structures determined here also are in good agreement with the auto-cleavage mechanism described for the flagellar homolog FlhB and E. coli EscU.
- Macromolecular Crystallography Laboratory, National Cancer Institute at Frederick, Frederick, Maryland 21702-1201, USA.
Organizational Affiliation: