A 1-Acetamido Derivative of 6-Epi-Valienamine: An Inhibitor of a Diverse Group of Beta-N-Acetylglucosaminidases.
Scaffidi, A., Stubbs, K.A., Dennis, R.J., Taylor, E.J., Davies, G.J., Vocadlo, D.J., Stick, R.V.(2007) Org Biomol Chem 5: 3013
- PubMed: 17728868
- DOI: https://doi.org/10.1039/b709681j
- Primary Citation of Related Structures:
2JIW - PubMed Abstract:
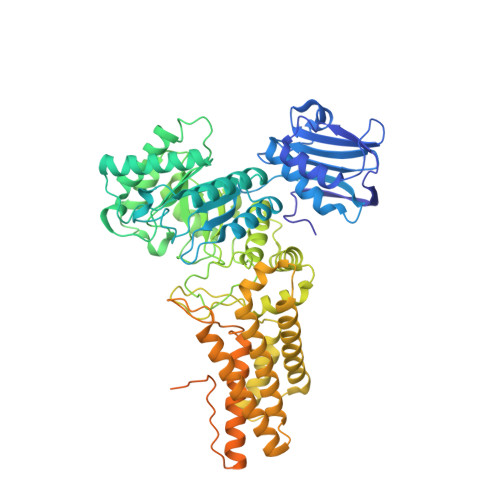
The synthesis of an analogue of 6-epi-valienamine bearing an acetamido group and its characterisation as an inhibitor of beta-N-acetylglucosaminidases are described. The compound is a good inhibitor of both human O-GlcNAcase and human beta-hexosaminidase, as well as two bacterial beta-N-acetylglucosaminidases. A 3-D structure of the complex of Bacteroides thetaiotaomicron BtGH84 with the inhibitor shows the unsaturated ring is surprisingly distorted away from its favoured solution phase conformation and reveals potential for improved inhibitor potency.
- Chemistry M313, School of Biomedical, Biomolecular and Chemical Sciences, The University of Western Australia, 35 Stirling Hwy, Crawley, WA, Australia.
Organizational Affiliation: