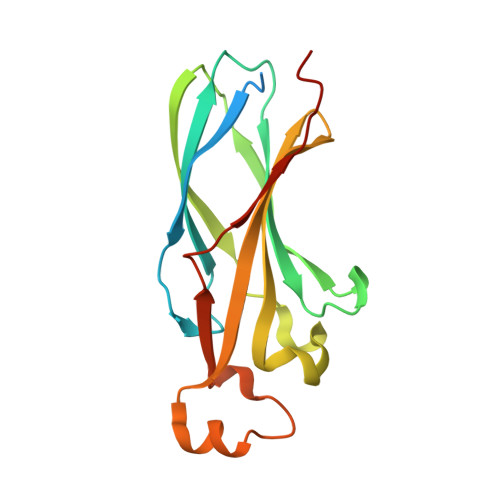
Structure of a human ASF1a-HIRA complex and insights into specificity of histone chaperone complex assembly.
Tang, Y., Poustovoitov, M.V., Zhao, K., Garfinkel, M., Canutescu, A., Dunbrack, R., Adams, P.D., Marmorstein, R.(2006) Nat Struct Mol Biol 13: 921-929
- PubMed: 16980972
- DOI: https://doi.org/10.1038/nsmb1147
- Primary Citation of Related Structures:
2I32 - PubMed Abstract:
Human HIRA, ASF1a, ASF1b and CAF-1 are evolutionally conserved histone chaperones that form multiple functionally distinct chromatin-assembly complexes, with roles linked to diverse nuclear process, such as DNA replication and formation of heterochromatin in senescent cells. We report the crystal structure of an ASF1a-HIRA heterodimer and a biochemical dissection of ASF1a's mutually exclusive interactions with HIRA and the p60 subunit of CAF-1. The HIRA B domain forms an antiparallel beta-hairpin that binds perpendicular to the strands of the beta-sandwich of ASF1a, via beta-sheet, salt bridge and van der Waals contacts. The N- and C-terminal regions of ASF1a and ASF1b determine the different affinities of these two proteins for HIRA, by contacting regions outside the HIRA B domain. CAF-1 p60 also uses B domain-like motifs for binding to ASF1a, thereby competing with HIRA. Together, these studies begin to define the molecular determinants of assembly of functionally diverse macromolecular histone chaperone complexes.
- The Wistar Institute, Philadelphia, Pennsylvania 19104, USA.
Organizational Affiliation: