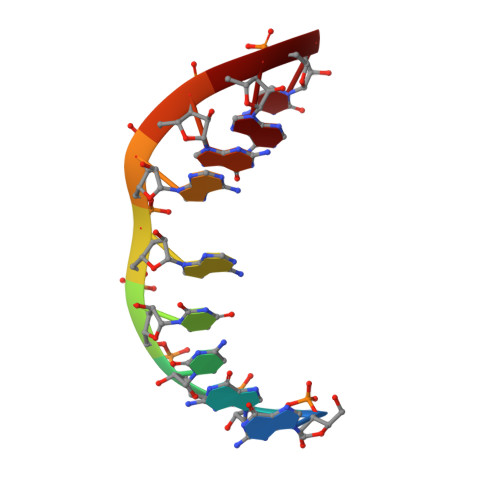
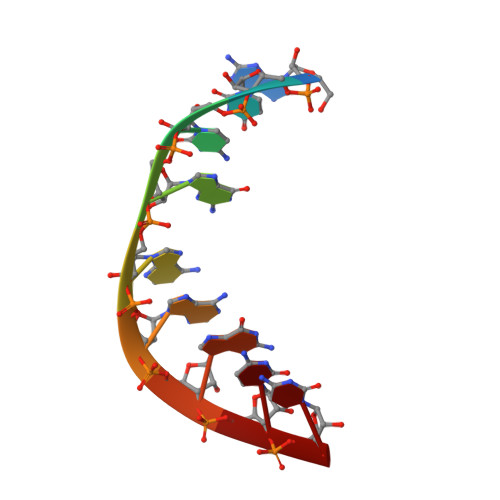
The NMR structure of an internal loop from 23S ribosomal RNA differs from its structure in crystals of 50s ribosomal subunits.
Shankar, N., Kennedy, S.D., Chen, G., Krugh, T.R., Turner, D.H.(2006) Biochemistry 45: 11776-11789
- PubMed: 17002278
- DOI: https://doi.org/10.1021/bi0605787
- Primary Citation of Related Structures:
2H49 - PubMed Abstract:
Internal loops play an important role in structure and folding of RNA and in recognition of RNA by other molecules such as proteins and ligands. An understanding of internal loops with propensities to form a particular structure will help predict RNA structure, recognition, and function. The structures of internal loops 5' 1009CUAAG1013 3'/3' 1168GAAGC1164 5' and 5' 998CUAAG1002 3'/3' 1157GAAGC1153 5' from helix 40 of the large subunit rRNA in Deinococcus radiodurans and Escherichia coli, respectively, are phylogenetically conserved, suggesting functional relevance. The energetics and NMR solution structure of the loop were determined in the duplex 5' 1GGCUAAGAC9 3'/3' 18CCGAAGCUG10 5'. The internal loop forms a different structure in solution and in the crystal structures of the ribosomal subunits. In particular, the crystal structures have a bulged out adenine at the equivalent of position A15 and a reverse Hoogsteen UA pair (trans Watson-Crick/Hoogsteen UA) at the equivalent of U4 and A14, whereas the solution structure has a single hydrogen bond UA pair (cis Watson-Crick/sugar edge A15U4) between U4 and A15 and a sheared AA pair (trans Hoogsteen/sugar edge A14A5) between A5 and A14. There is cross-strand stacking between A6 and A14 (A6/A14/A15 stacking pattern) in the NMR structure. All three structures have a sheared GA pair (trans Hoogsteen/sugar edge A6G13) at the equivalent of A6 and G13. The internal loop has contacts with ribosomal protein L20 and other parts of the RNA in the crystal structures. These contacts presumably provide the free energy to rearrange the base pairing in the loop. Evidently, molecular recognition of this internal loop involves induced fit binding, which could confer several advantages. The predicted thermodynamic stability of the loop agrees with the experimental value, even though the thermodynamic model assumes a Watson-Crick UA pair.
- Department of Biochemistry and Biophysics, School of Medicine and Dentistry, University of Rochester, Rochester, New York 14642, USA.
Organizational Affiliation: