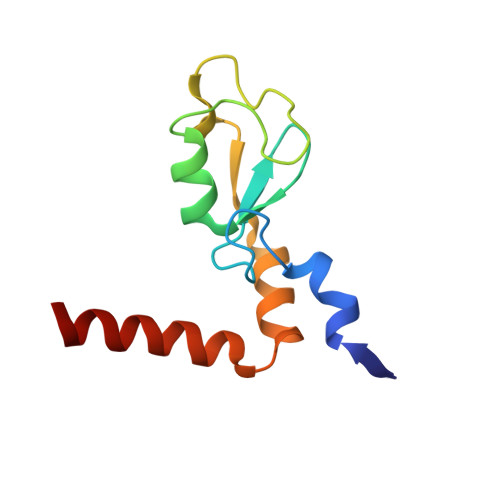
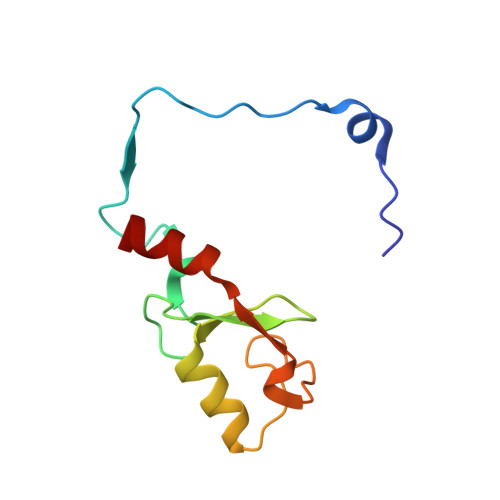
Structure of a Bmi-1-Ring1B Polycomb Group Ubiquitin Ligase Complex.
Li, Z., Cao, R., Wang, M., Myers, M.P., Zhang, Y., Xu, R.M.(2006) J Biological Chem 281: 20643-20649
- PubMed: 16714294
- DOI: https://doi.org/10.1074/jbc.M602461200
- Primary Citation of Related Structures:
2H0D - PubMed Abstract:
Polycomb group proteins Bmi-1 and Ring1B are core subunits of the PRC1 complex, which plays important roles in the regulation of Hox gene expression, X-chromosome inactivation, tumorigenesis, and stem cell self-renewal. The RING finger protein Ring1B is an E3 ligase that participates in the ubiquitination of lysine 119 of histone H2A, and the binding of Bmi-1 stimulates the E3 ligase activity. We have mapped the regions of Bmi-1 and Ring1B required for efficient ubiquitin transfer and determined a 2.5-A structure of the Bmi-1-Ring1B core domain complex. The structure reveals that Ring1B "hugs" Bmi-1 through extensive RING domain contacts and its N-terminal tail wraps around Bmi-1. The two regions of interaction have a synergistic effect on the E3 ligase activity. Our analyses suggest a model where the Bmi-1-Ring1B complex stabilizes the interaction between the E2 enzyme and the nucleosomal substrate to allow efficient ubiquitin transfer.
- Cold Spring Harbor Laboratory, Cold Spring Harbor, New York 11724, USA.
Organizational Affiliation: