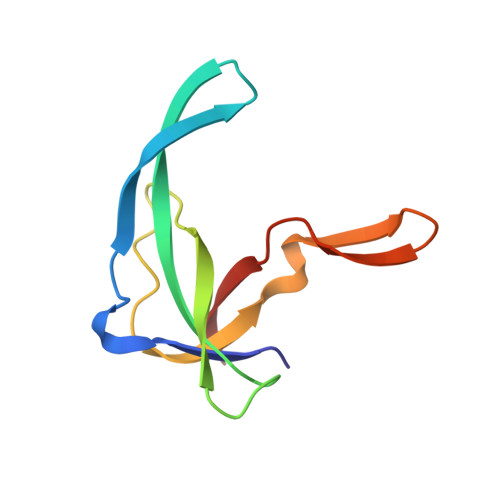
Refined solution structure of the Tyr41-->His mutant of the M13 gene V protein. A comparison with the crystal structure.
Prompers, J.J., Folmer, R.H., Nilges, M., Folkers, P.J., Konings, R.N., Hilbers, C.W.(1995) Eur J Biochem 232: 506-514
- PubMed: 7556200
- Primary Citation of Related Structures:
2GVA, 2GVB - PubMed Abstract:
The three-dimensional solution structure of mutant Tyr41-->His of the single-stranded DNA binding protein encoded by gene V of the filamentous bacteriophage M13 has been refined in two stages. The first stage involved the collection of additional NOE-based distance constraints, which were then used in eight cycles of back-calculations and structure calculations. The structures of the gene V protein dimers were calculated using simulated annealing, employing restrained molecular dynamics with a geometric force field. In the second stage of the refinement procedure, solvent was explicitly included during the dynamic calculations. A total of 30 structures was calculated for the protein, representing its solution structure in water. The first calculation step significantly improved the convergence of the structures, whereas the subsequent simulations in water made the structures physically more realistic. This is, for instance, illustrated by the number of hydrogen bonds formed in the molecule, which increased considerably upon going to aqueous solution. It is shown that the solution structure of the mutant gene V protein is nearly identical to the crystal structure of the wild-type molecule, except for the DNA-binding loop (residues 16-28). This antiparallel beta-hairpin is twisted and partially folded back towards the core of the protein in the NMR structure, whereas it is more extended and points away from the rest of the molecule in the X-ray structure. Unrestrained molecular dynamics calculations suggest that this latter conformation is energetically unstable in solution.
- Nijmegen Son Research Center, University of Nijmegen, The Netherlands.
Organizational Affiliation: