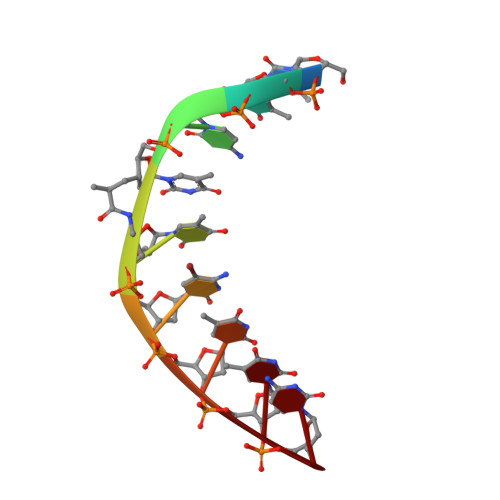
RNA-Binding Affinities and Crystal Structure of Oligonucleotides Containing Five-Atom Amide-Based Backbone Structures.
Pallan, P.S., von Matt, P., Wilds, C.J., Altmann, K.-H., Egli, M.(2006) Biochemistry 45: 8048-8057
- PubMed: 16800629
- DOI: https://doi.org/10.1021/bi060354o
- Primary Citation of Related Structures:
2GUN - PubMed Abstract:
Among the hundreds of nucleic acid analogues that have been studied over the last two decades only very few exhibit backbones with linkers between residues that are either shorter or longer than the four-atom linker O3'-P-O5'-C5' connecting sugar ring moieties in DNA and RNA. 2'-Deoxyribonucleoside dimers connected by a five-atom linker O3'-CH(CH(3))-CO-NH-CH(2) (*designates a chiral center) were reported to lead to only a slight destabilization of RNA-DNA hybrids in which the DNA strand contained one or several of these amide-linked dimers (De Napoli, L., Iadonisi, A., Montesarchio, D., Varra, M., and Piccialli, G. (1995) Synthesis of thymidine dimers containing a new internucleosidic amide linkage and their incorporation into oligodeoxyribonucleotides, Bioorg. Med. Chem. Lett. 5, 1647-1652). To analyze the influence of various chemistries of such five-atom amide linkers on the RNA-binding affinity of modified DNA strands, we have synthesized five different amide-linked dimers, including structures with homochiral linkers of the type X3'-C*H(CH(3))-CO-NH-CH(2) (X = O, CH(2)) as well as the corresponding analogues carrying methoxy groups at the 2'-position of the 3'-nucleosides. We have conducted a detailed thermodynamic analysis of duplex formation between the modified DNA and RNA, with the DNA strands containing between one and seven consecutive modified dimers. Some of the five-atom-linked dimers lead to significantly higher RNA-binding affinities compared with that of native DNA. Interestingly, the linkers with opposite stereochemistry at the chiral center stabilize duplexes between the modified DNA and RNA to different degrees. CD spectroscopy in solution and a crystal structure of an RNA-DNA duplex with a single amide-linked dimer demonstrate that the longer amide backbones do not disrupt the duplex geometry. These observations provide further evidence that stable cross-pairing between two different types of nucleic acids does not require the numbers of atoms linking their individual residues to match.
- Department of Biochemistry, School of Medicine, Vanderbilt University, Nashville, Tennessee 37232, USA.
Organizational Affiliation: