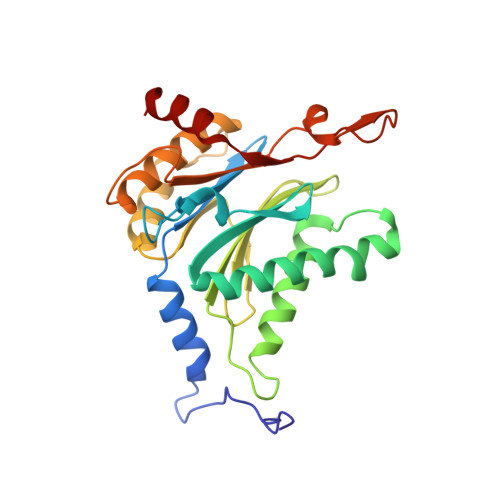
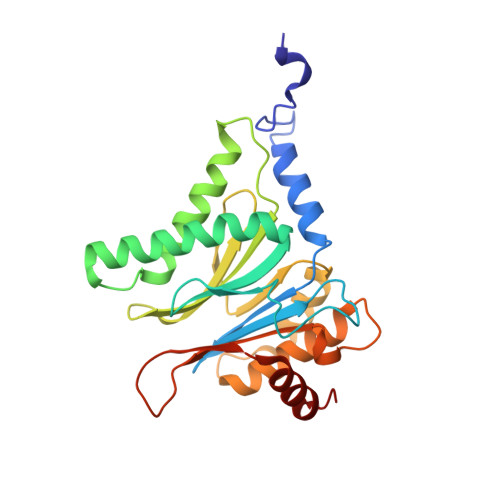
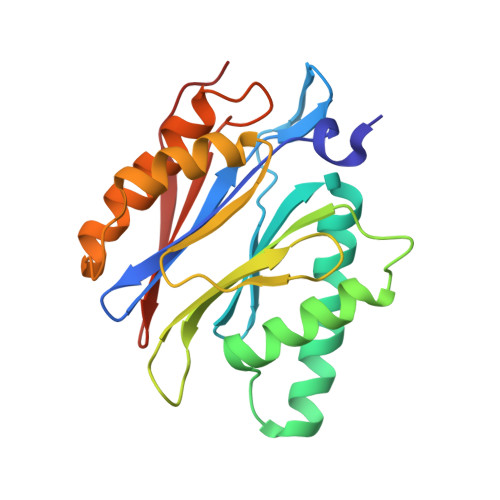
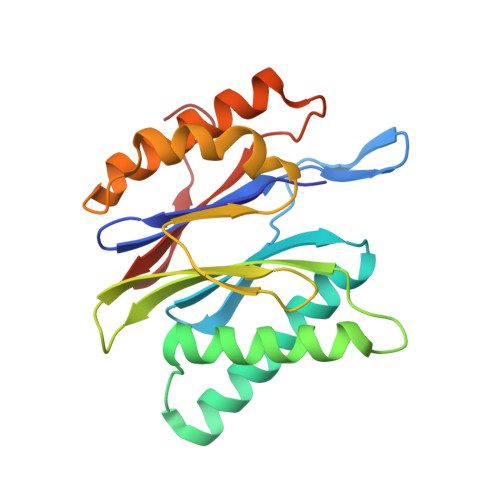
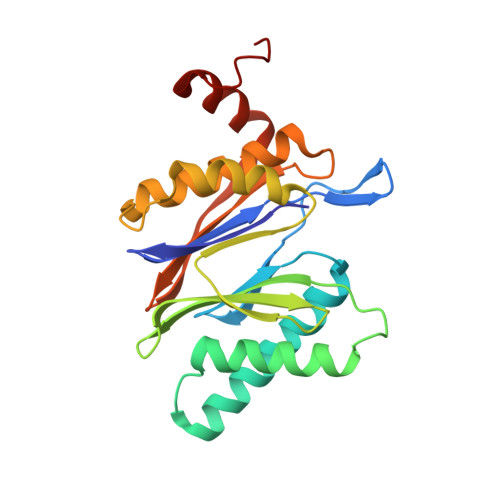
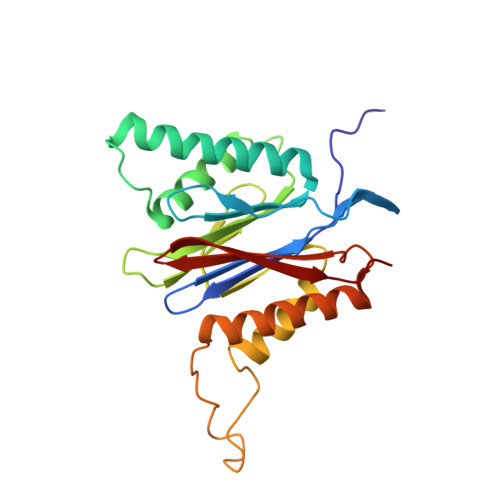
TMC-95-Based Inhibitor Design Provides Evidence for the Catalytic Versatility of the Proteasome.
Groll, M., Goetz, M., Kaiser, M., Weyher, E., Moroder, L.(2006) Chem Biol 13: 607-614
- PubMed: 16793518
- DOI: https://doi.org/10.1016/j.chembiol.2006.04.005
- Primary Citation of Related Structures:
2GPL - PubMed Abstract:
TMC-95's natural cyclic tripeptide metabolites represent potent competitive proteasome inhibitors. The constrained conformation of TMC-95 proteasomal inhibitors provides the driving force for entropically high-affinity binding. Based on the crystal structure of the proteasome:TMC-95A complex, the synthetically challenging TMC-95 core structure was used for the design and synthesis of less demanding biphenyl-ether macrocycles, in which the biphenyl-ether moiety functions as an endocyclic clamp restricting its tripeptide backbone. These simplified analogs allowed us to identify high plasticity of the proteasomal tryptic-like specificity pocket. Biphenyl-ether compounds extended with an amide group were hydrolyzed by the proteasome, although the crystal structure of such proteasome:biphenyl-ether complexes revealed quenching of proteolysis at the acyl-enzyme intermediate. Our data reveal that biphenyl-ether derivatives bind noncovalently to the proteasomal tryptic-like active site in a reversible substrate-like manner without allosteric changes of active site residues.
- Ludwig-Maximilians-University, Department for Physiological Chemistry, Butenandtstrasse 5, Building B, D-81377 Munich, Germany. mgroll@med.uni-muenxhen.de
Organizational Affiliation: