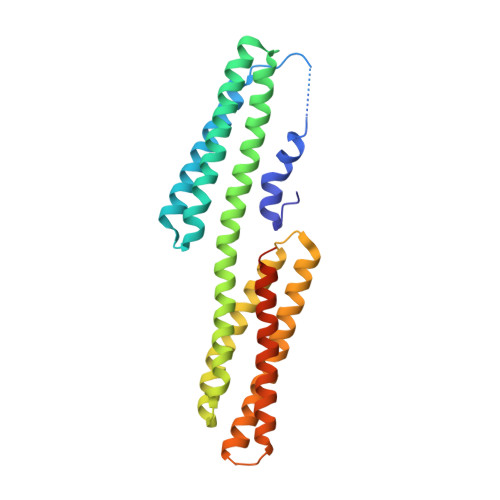
Structural mimicry for vinculin activation by IpaA, a virulence factor of Shigella flexneri.
Hamiaux, C., van Eerde, A., Parsot, C., Broos, J., Dijkstra, B.W.(2006) EMBO Rep 7: 794-799
- PubMed: 16826238
- DOI: https://doi.org/10.1038/sj.embor.7400753
- Primary Citation of Related Structures:
2GDC - PubMed Abstract:
Invasion of epithelial cells by Shigella flexneri is characterized by cytoskeletal rearrangements of the host cell membrane, promoting internalization of the bacterium. The bacterial effector IpaA is injected into the epithelial cell by a type III secretion apparatus and recruits vinculin to regulate actin polymerization at the site of entry. We analysed the complex formed between a carboxy-terminal fragment of IpaA (IpaA(560-633)) and the vinculin D1 domain (VD1), both in crystals and in solution. We present evidence that IpaA(560-633) has two alpha-helical vinculin-binding sites that simultaneously bind two VD1 molecules. The interaction of IpaA(560-633) with VD1 is highly similar to the interaction of the endogenous, eukaryotic proteins talin and alpha-actinin with VD1, showing that Shigella uses a structural mimicry strategy to activate vinculin.
- Laboratory of Biophysical Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands.
Organizational Affiliation: