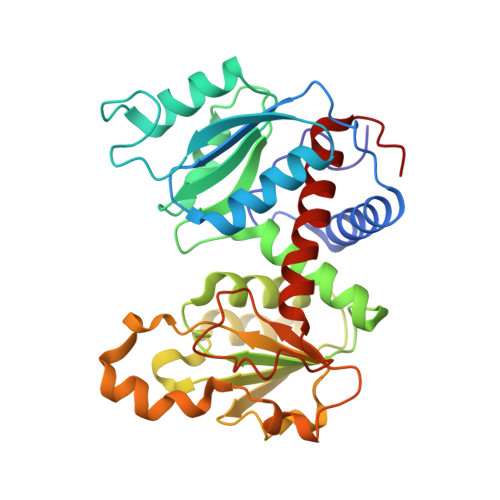
T-state Inhibitors of E. coli Aspartate Transcarbamoylase that Prevent the Allosteric Transition.
Heng, S., Stieglitz, K.A., Eldo, J., Xia, J., Cardia, J.P., Kantrowitz, E.R.(2006) Biochemistry 45: 10062-10071
- PubMed: 16906764
- DOI: https://doi.org/10.1021/bi0601095
- Primary Citation of Related Structures:
2FZC, 2FZG, 2FZK - PubMed Abstract:
Escherichia coli aspartate transcarbamoylase (ATCase) catalyzes the committed step in pyrimidine nucleotide biosynthesis, the reaction between carbamoyl phosphate (CP) and l-aspartate to form N-carbamoyl-l-aspartate and inorganic phosphate. The enzyme exhibits homotropic cooperativity and is allosterically regulated. Upon binding l-aspartate in the presence of a saturating concentration of CP, the enzyme is converted from the low-activity low-affinity T state to the high-activity high-affinity R state. The potent inhibitor N-phosphonacetyl-l-aspartate (PALA), which combines the binding features of Asp and CP into one molecule, has been shown to induce the allosteric transition to the R state. In the presence of only CP, the enzyme is the T structure with the active site primed for the binding of aspartate. In a structure of the enzyme-CP complex (T(CP)), two CP molecules were observed in the active site approximately 7A apart, one with high occupancy and one with low occupancy. The high occupancy site corresponds to the position for CP observed in the structure of the enzyme with CP and the aspartate analogue succinate bound. The position of the second CP is in a unique site and does not overlap with the aspartate binding site. As a means to generate a new class of inhibitors for ATCase, the domain-open T state of the enzyme was targeted. We designed, synthesized, and characterized three inhibitors that were composed of two phosphonacetamide groups linked together. These two phosphonacetamide groups mimic the positions of the two CP molecules in the T(CP) structure. X-ray crystal structures of ATCase-inhibitor complexes revealed that each of these inhibitors bind to the T state of the enzyme and occupy the active site area. As opposed to the binding of Asp in the presence of CP or PALA, these inhibitors are unable to initiate the global T to R conformational change. Although the best of these T-state inhibitors only has a K(i) value in the micromolar range, the structural information with respect to their mode of binding provides important information for the design of second generation inhibitors that will have even higher affinity for the active site of the T state of the enzyme.
- Department of Chemistry, Merkert Chemistry Center, Boston College, Chestnut Hill, Massachusetts 02467, USA.
Organizational Affiliation: