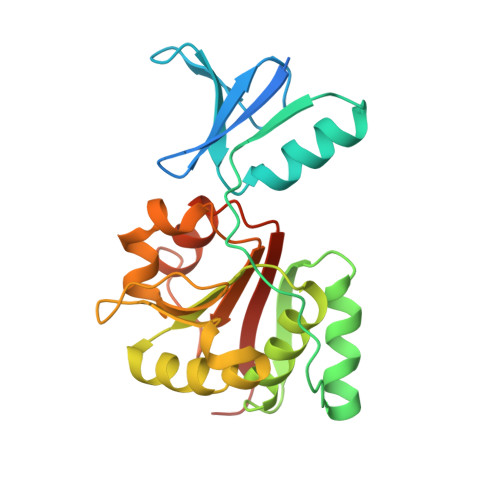
Conserved XPB Core Structure and Motifs for DNA Unwinding: Implications for Pathway Selection of Transcription or Excision Repair.
Fan, L., Arvai, A.S., Cooper, P.K., Iwai, S., Hanaoka, F., Tainer, J.A.(2006) Mol Cell 22: 27-37
- PubMed: 16600867
- DOI: https://doi.org/10.1016/j.molcel.2006.02.017
- Primary Citation of Related Structures:
2FWR, 2FZ4, 2FZL - PubMed Abstract:
The human xeroderma pigmentosum group B (XPB) helicase is essential for transcription, nucleotide excision repair, and TFIIH functional assembly. Here, we determined crystal structures of an Archaeoglobus fulgidus XPB homolog (AfXPB) that characterize two RecA-like XPB helicase domains and discover a DNA damage recognition domain (DRD), a unique RED motif, a flexible thumb motif (ThM), and implied conformational changes within a conserved functional core. RED motif mutations dramatically reduce helicase activity, and the DRD and ThM, which flank the RED motif, appear structurally as well as functionally analogous to the MutS mismatch recognition and DNA polymerase thumb domains. Substrate specificity is altered by DNA damage, such that AfXPB unwinds dsDNA with 3' extensions, but not blunt-ended dsDNA, unless it contains a lesion, as shown for CPD or (6-4) photoproducts. Together, these results provide an unexpected mechanism of DNA unwinding with implications for XPB damage verification in nucleotide excision repair.
- Life Sciences Division, Department of Molecular Biology, Lawrence Berkeley National Laboratory, Berkeley, California 94720, USA.
Organizational Affiliation: