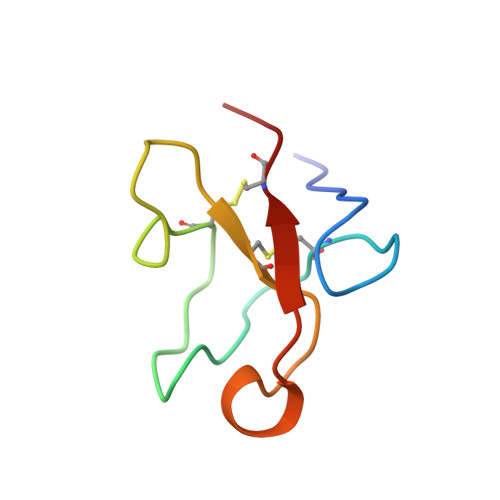
Solution structure of the glycosylated second type 2 module of fibronectin.
Sticht, H., Pickford, A.R., Potts, J.R., Campbell, I.D.(1998) J Mol Biology 276: 177-187
- PubMed: 9514732
- DOI: https://doi.org/10.1006/jmbi.1997.1528
- Primary Citation of Related Structures:
2FN2 - PubMed Abstract:
Fibronectin is an extracellular matrix glycoprotein that plays a role in a number of physiological processes involving cell adhesion and migration. The modules of the fibronectin monomer are organized into proteolytically resistant domains that in isolation retain their affinity for various ligands. The tertiary structure of the glycosylated second type 2 module (2F2) from the gelatin-binding domain of fibronectin was determined by two-dimensional nuclear magnetic resonance spectroscopy and simulated annealing. The structure is well defined with an overall fold typical of F2 modules, showing two double-stranded antiparallel beta-sheets and a partially solvent-exposed hydrophobic cluster. An N-terminal beta-sheet, that was not present in previously determined F2 module structures, may be important for defining the relative orientation of adjacent F2 modules in fibronectin. This is the first three-dimensional structure of a glycosylated module of fibronectin, and provides insight into the possible role of the glycosylation in protein stability, protease resistance and modulation of collagen binding. Based on the structures of the isolated modules, models for the 1F22F2 pair were generated by randomly changing the orientation of the linker peptide between the modules. The models suggest that the two putative collagen binding sites in the pair form discrete binding sites, rather than combining to form a single binding site.
- Department of Biochemistry University of Oxford, UK.
Organizational Affiliation: