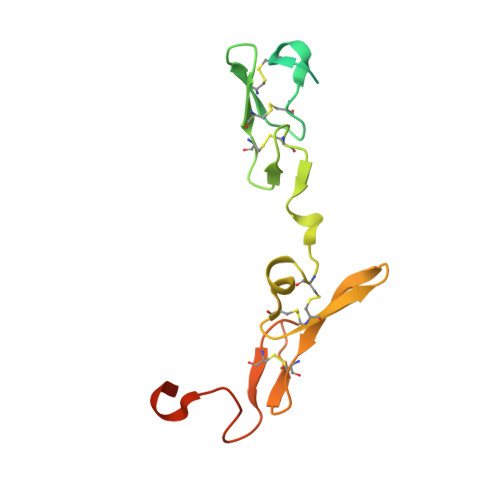
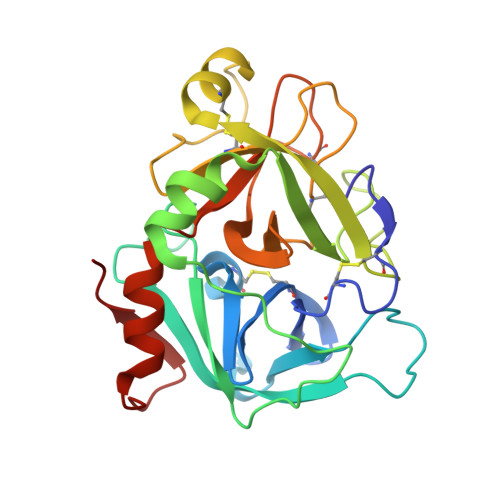
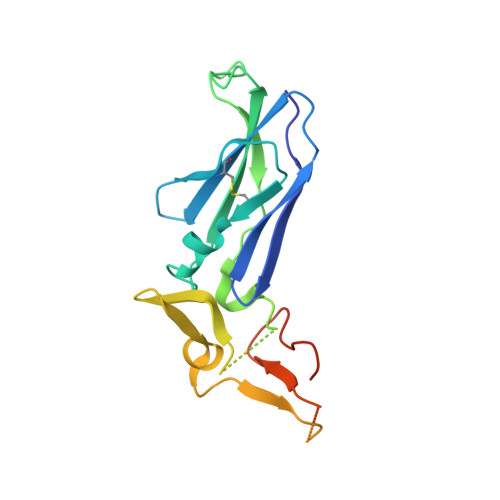
Discovery of novel hydroxy pyrazole based factor IXa inhibitor.
Vijaykumar, D., Sprengeler, P.A., Shaghafi, M., Spencer, J.R., Katz, B.A., Yu, C., Rai, R., Young, W.B., Schultz, B., Janc, J.(2006) Bioorg Med Chem Lett 16: 2796-2799
- PubMed: 16487703
- DOI: https://doi.org/10.1016/j.bmcl.2006.01.123
- Primary Citation of Related Structures:
2FLB - PubMed Abstract:
Synthesis and biological data of a novel selective and efficacious factor IXa inhibitor are described along with its crystal structure in factor VIIa.
- Celera, 180 Kimball Way, South San Francisco, CA 94080, USA. dvijayku@hotmail.com
Organizational Affiliation: