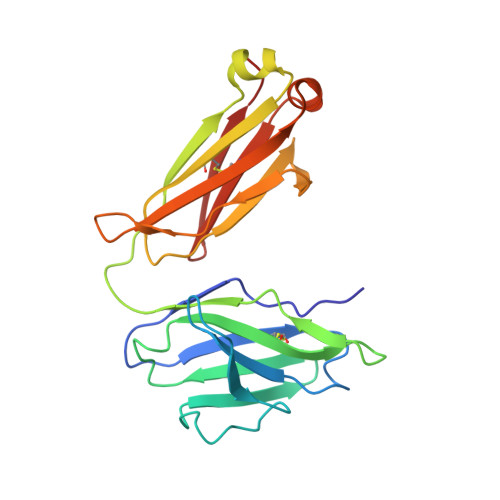
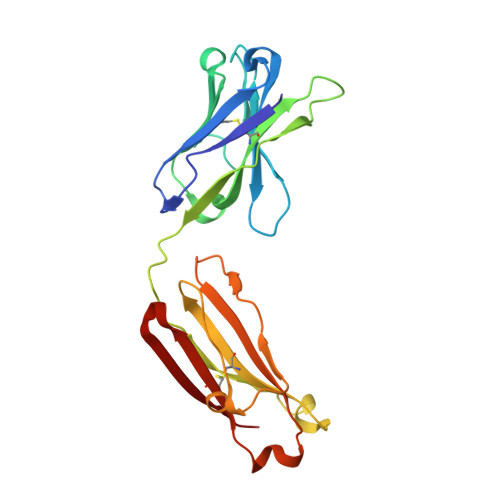
Cofactor-containing antibodies: Crystal structure of the original yellow antibody.
Zhu, X., Wentworth, P., Kyle, R.A., Lerner, R.A., Wilson, I.A.(2006) Proc Natl Acad Sci U S A 103: 3581-3585
- PubMed: 16537445
- DOI: https://doi.org/10.1073/pnas.0600251103
- Primary Citation of Related Structures:
2FL5 - PubMed Abstract:
Antibodies are generally thought to be a class of proteins that function without the use of cofactors. However, it is not widely appreciated that antibodies are believed to be the major carrier protein in human circulation for the important riboflavin cofactor that is involved in a host of biological phenomena. A further link between riboflavin and antibodies was discovered 30 years ago when a bright-yellow antibody, IgG(GAR), was purified from a patient with multiple myeloma who had turned yellow during the course of her disease. It was subsequently shown that the yellow color of this antibody was due to riboflavin binding. However, it was not known how and where riboflavin was bound to this antibody. We now report the crystal structure of this historically important IgG(GAR) Fab at 3.0-A resolution. The riboflavin is located in the antigen-combining site with its isoalloxazine ring stacked between the parallel aromatic moieties of TyrH33, PheH58, and TyrH100A. Together with additional hydrogen bonds, these interactions reveal the structural basis for high-affinity riboflavin binding. The ligand specificity of IgG(GAR) is compared with another riboflavin-binding antibody, IgG(DOT), which was purified from a second patient with multiple myeloma. The crystal structure of IgG(GAR) provides a starting point for attempts to understand the physiological relevance and chemical functions of cofactor-containing antibodies.
- Department of Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA.
Organizational Affiliation: