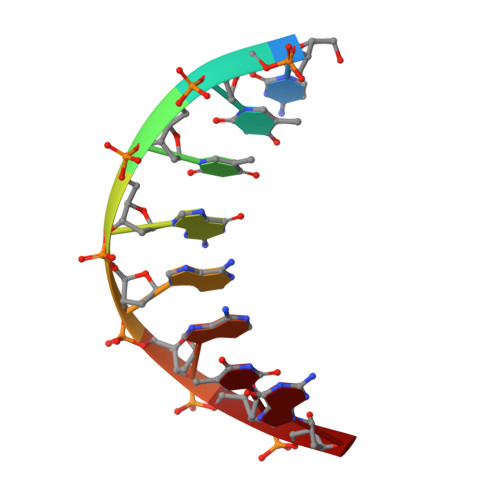
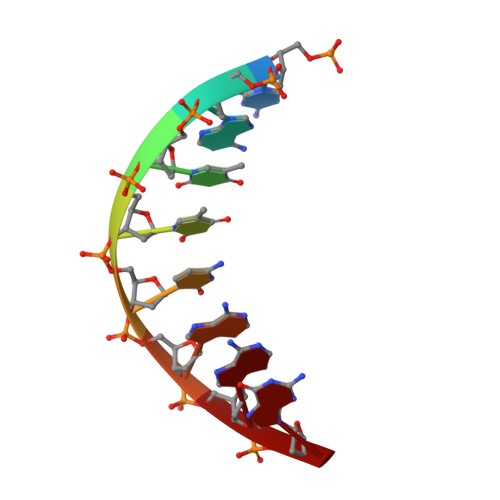
A High-Throughput, High-Resolution Strategy for the Study of Site-Selective DNA Binding Agents: Analysis of a "Highly Twisted" Benzimidazole-Diamidine.
Goodwin, K.D., Lewis, M.A., Tanious, F.A., Tidwell, R.R., Wilson, W.D., Georgiadis, M.M., Long, E.C.(2006) J Am Chem Soc 128: 7846-7854
- PubMed: 16771498
- DOI: https://doi.org/10.1021/ja0600936
- Primary Citation of Related Structures:
2FJV, 2FJW, 2FJX - PubMed Abstract:
A general strategy for the rapid structural analysis of DNA binding ligands is described as it was applied to the study of RT29, a benzimidazole-diamidine compound containing a highly twisted diphenyl ether linkage. By combining the existing high-throughput fluorescent intercalator displacement (HT-FID) assay developed by Boger et al. and a high-resolution (HR) host-guest crystallographic technique, a system was produced that was capable of determining detailed structural information pertaining to RT29-DNA interactions within approximately 3 days. Our application of the HT/HR strategy immediately revealed that RT29 has a preference for 4-base pair (bp), A.T-rich sites (AATT) and a similar tolerance and affinity for three A-T-bp sites (such as ATTC) containing a G.C bp. On the basis of these selectivities, oligonucleotides were designed and the host-guest crystallographic method was used to generate diffraction quality crystals. Analysis of the resulting crystal structures revealed that the diphenyl ether moiety of RT29 undergoes conformational changes that allow it to adopt a crescent shape that now complements the minor groove structure. The presence of a G.C bp in the RT29 binding site of ATTC did not overly perturb its interaction with DNA-the compound adjusted to the nucleobases that were available through water-mediated interactions. Our analyses suggest that the HT/HR strategy may be used to expedite the screening of novel minor groove binding compounds leading to a direct, HR structural determination.
- Department of Biochemistry & Molecular Biology, Indiana University School of Medicine, Purdue School of Science, Indiana University-Purdue University Indianapolis (IUPUI), Indianapolis, Indiana 46202, USA.
Organizational Affiliation: