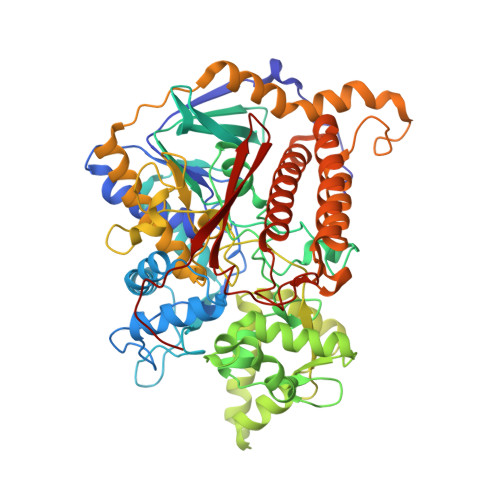
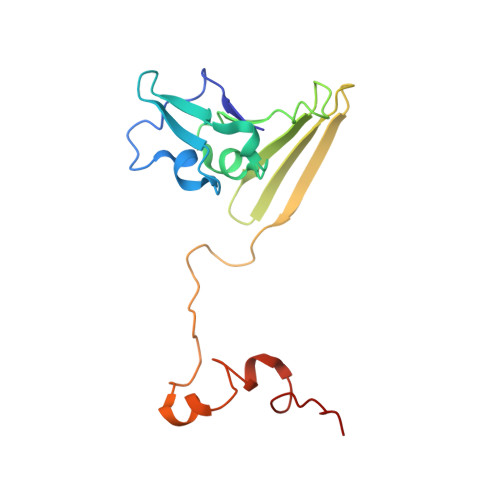
Reaction mechanism of the iron-sulfur flavoenzyme adenosine-5'-phosphosulfate reductase based on the structural characterization of different enzymatic states
Schiffer, A., Fritz, G., Kroneck, P.M., Ermler, U.(2006) Biochemistry 45: 2960-2967
- PubMed: 16503650
- DOI: https://doi.org/10.1021/bi0521689
- Primary Citation of Related Structures:
2FJA, 2FJB, 2FJD, 2FJE - PubMed Abstract:
The iron-sulfur flavoenzyme adenosine-5'-phosphosulfate (APS) reductase catalyzes a key reaction of the global sulfur cycle by reversibly transforming APS to sulfite and AMP. The structures of the dissimilatory enzyme from Archaeoglobus fulgidus in the reduced state (FAD(red)) and in the sulfite adduct state (FAD-sulfite-AMP) have been recently elucidated at 1.6 and 2.5 A resolution, respectively. Here we present new structural features of the enzyme trapped in four different catalytically relevant states that provide us with a detailed picture of its reaction cycle. In the oxidized state (FAD(ox)), the isoalloxazine moiety of the FAD cofactor exhibits a similarly bent conformation as observed in the structure of the reduced enzyme. In the APS-bound state (FAD(ox)-APS), the substrate APS is embedded into a 17 A long substrate channel in such a way that the isoalloxazine ring is pushed toward the channel bottom, thereby producing a compressed enzyme-substrate complex. A clamp formed by residues ArgA317 and LeuA278 to fix the adenine ring and the curved APS conformation appear to be key factors to hold APS in a strained conformation. This energy-rich state is relaxed during the attack of APS on the reduced FAD. A relaxed FAD-sulfite adduct is observed in the structure of the FAD-sulfite state. Finally, a FAD-sulfite-AMP1 state with AMP within van der Waals distance of the sulfite adduct could be characterized. This structure documents how adjacent negative charges are stabilized by the protein matrix which is crucial for forming APS from AMP and sulfite in the reverse reaction.
- Fachbereich Biologie, Mathematisch-Naturwissenschaftliche Sektion, Universität Konstanz, D-78457 Konstanz, Germany.
Organizational Affiliation: