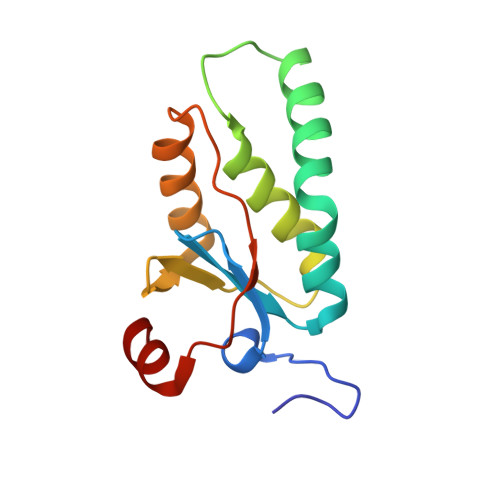
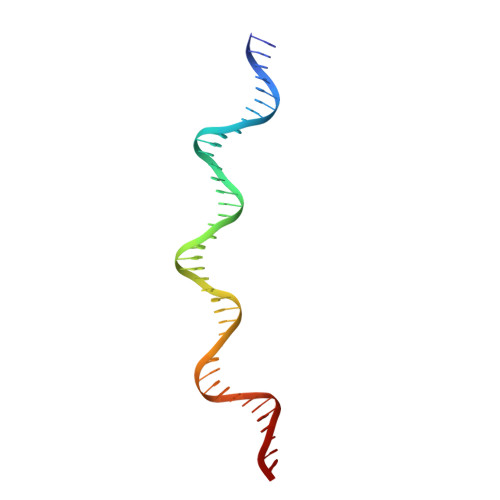
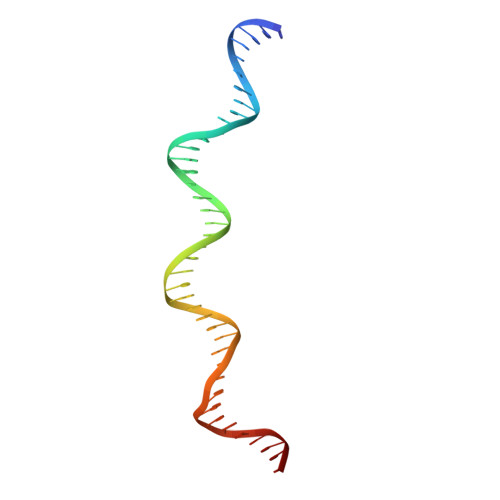
The structure of phage phi29 transcription regulator p4-DNA complex reveals an N-hook motif for DNA
Badia, D., Camacho, A., Perez-Lago, L., Escandon, C., Salas, M., Coll, M.(2006) Mol Cell 22: 73-81
- PubMed: 16600871
- DOI: https://doi.org/10.1016/j.molcel.2006.02.019
- Primary Citation of Related Structures:
2FIO, 2FIP - PubMed Abstract:
Protein p4 affects the transcriptional switch that divides bacteriophage phi29 infection in early and late phases. The synthesis of DNA replication proteins and p4 takes place in the early phase, while structural, morphogenesis, and lysis proteins are synthesized in the late phase. Transcriptional switch by p4 is achieved by activating the late promoter A3 and repressing the early promoters A2b and A2c. The crystal structure of p4 alone and in complex with a 41 bp DNA, including the A3 promoter binding site, helps us to understand how the phage cycle is controlled. Protein p4 has a unique alpha/beta fold that includes a DNA recognition motif consisting of two N-terminal beta turn substructures, or N-hooks, located at the tips of an elongated protein homodimer. The two N-hooks enter the major groove of the double helix, establishing base-specific contacts. A high DNA curvature allows p4 N-hooks to reach two major groove areas three helical turns apart, like a bow and its string.
- Institut de Biologia Molecular de Barcelona (CSIC) and Institut de Recerca Biomèdica, Parc Científic de Barcelona, Josep Samitier 1-5, 08028 Barcelona, Spain.
Organizational Affiliation: